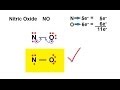
Nitrous Oxide Lewis Structure Concepts
Interactive Video
•
Chemistry, Science, Biology
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a key reason for exceptions to the octet rule in elements from the third or fourth period?
They are less reactive.
They are more electronegative.
They have fewer than eight valence electrons.
They have more than eight valence electrons.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is nitrous oxide unable to satisfy the octet rule?
It has an odd number of valence electrons.
It has an even number of valence electrons.
It is too stable.
It is too reactive.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in nitrous oxide?
Ten
Thirteen
Twelve
Eleven
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the initial Lewis structure attempt for nitrous oxide, how many valence electrons are around the nitrogen atom?
Four
Six
Five
Seven
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of electronegativity in determining the central atom in a Lewis structure?
The most electronegative atom is central.
The least electronegative atom is central.
The central atom is always oxygen.
Electronegativity does not affect the central atom.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens when a double bond is introduced in the Lewis structure of nitrous oxide?
Both atoms follow the octet rule.
Oxygen follows the octet rule.
Neither atom follows the octet rule.
Nitrogen follows the octet rule.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom in nitrous oxide is more electronegative?
Nitrogen
Oxygen
Both are equally electronegative
Neither is electronegative
Create a free account and access millions of resources
Similar Resources on Wayground

9 questions
Strontium and Oxygen Chemistry Concepts
Interactive video
•
9th - 10th Grade

11 questions
Ionic Compounds and Bonding Concepts
Interactive video
•
9th - 10th Grade

11 questions
Ionic Compounds and Electron Configuration
Interactive video
•
9th - 10th Grade

9 questions
Valence Electrons and Octet Rule
Interactive video
•
9th - 10th Grade

7 questions
Valence Electrons in IBr2- Structure
Interactive video
•
9th - 10th Grade

8 questions
Carbon Bonding and Valence Electrons
Interactive video
•
9th - 10th Grade

9 questions
Valence Electrons and Molecular Structure
Interactive video
•
9th - 10th Grade

10 questions
Beryllium and Oxygen Compounds
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade