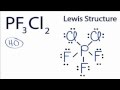
PF3Cl2 Lewis Structure Concepts
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in the PF3Cl2 molecule?
36
48
32
40
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is placed at the center of the PF3Cl2 Lewis structure?
Oxygen
Phosphorus
Chlorine
Fluorine
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to form chemical bonds in the PF3Cl2 structure initially?
14
8
10
12
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of placing lines between atoms in the Lewis structure?
To represent lone pairs
To show molecular geometry
To form chemical bonds
To indicate ionic bonds
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does phosphorus have in the PF3Cl2 structure?
8
10
12
14
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it acceptable for phosphorus to have more than 8 valence electrons in PF3Cl2?
It is a noble gas
It is in period 3 and can have an expanded octet
It forms ionic bonds
It is highly electronegative
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formal charge of each atom in the PF3Cl2 Lewis structure?
+2
0
-1
+1
Similar Resources on Wayground

6 questions
C2H2Br2 Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

6 questions
Lewis Structure of Methanol
Interactive video
•
9th - 10th Grade

7 questions
Valence Electrons in N2H4
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in IBr Molecule
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
Lewis Structures and Molecular Geometry
Interactive video
•
9th - 10th Grade

6 questions
SF6 Lewis Structure and Valence Electrons
Interactive video
•
9th - 10th Grade

7 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
Molecules, Compounds, & Elements
Quiz
•
9th Grade