How many valence electrons does Silicon have?
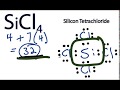
Valence Electrons in SiCl4
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Mia Campbell
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
8
6
4
2
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in SiCl4?
34
32
30
28
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is placed at the center of the SiCl4 molecule?
Silicon
Chlorine
Hydrogen
Oxygen
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to form bonds between Silicon and Chlorine?
4
6
8
10
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Does each atom in SiCl4 satisfy the octet rule?
Yes, all atoms have a full outer shell.
No, none of the atoms satisfy the octet rule.
No, only Chlorine atoms satisfy the octet rule.
No, only Silicon satisfies the octet rule.
Similar Resources on Wayground

6 questions
Valence Electrons in SiBr4
Interactive video
•
9th - 10th Grade

6 questions
Lewis Structure and Valence Electrons
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in CBr4
Interactive video
•
9th - 10th Grade

6 questions
Lewis Structures and Valence Electrons
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in PCl3
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in CH2F2
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

7 questions
Valence Electrons in SeCl4
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

25 questions
Equations of Circles
Quiz
•
10th - 11th Grade

30 questions
Week 5 Memory Builder 1 (Multiplication and Division Facts)
Quiz
•
9th Grade

33 questions
Unit 3 Summative - Summer School: Immune System
Quiz
•
10th Grade

10 questions
Writing and Identifying Ratios Practice
Quiz
•
5th - 6th Grade

36 questions
Prime and Composite Numbers
Quiz
•
5th Grade

14 questions
Exterior and Interior angles of Polygons
Quiz
•
8th Grade

37 questions
Camp Re-cap Week 1 (no regression)
Quiz
•
9th - 12th Grade

46 questions
Biology Semester 1 Review
Quiz
•
10th Grade
Discover more resources for Chemistry

25 questions
Equations of Circles
Quiz
•
10th - 11th Grade

30 questions
Week 5 Memory Builder 1 (Multiplication and Division Facts)
Quiz
•
9th Grade

33 questions
Unit 3 Summative - Summer School: Immune System
Quiz
•
10th Grade

37 questions
Camp Re-cap Week 1 (no regression)
Quiz
•
9th - 12th Grade

46 questions
Biology Semester 1 Review
Quiz
•
10th Grade