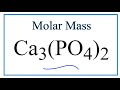
Molar Mass and Atomic Structure
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Emma Peterson
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic mass of calcium as mentioned in the video?
40.08 grams per mole
12.01 grams per mole
30.97 grams per mole
16.00 grams per mole
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many phosphorus atoms are present in calcium phosphate?
4
3
2
1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic mass of oxygen used in the calculation?
18.00 grams per mole
14.01 grams per mole
12.01 grams per mole
16.00 grams per mole
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many times is the phosphate ion multiplied in the calculation?
1
2
3
4
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final molar mass of calcium phosphate calculated in the video?
320.18 grams per mole
330.18 grams per mole
300.18 grams per mole
310.18 grams per mole
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why might your calculated molar mass differ slightly from the video's result?
Miscounted atoms
Incorrect multiplication
Different periodic table precision
Wrong chemical formula
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the number of decimal places in the periodic table?
It alters the chemical properties
It affects the precision of the molar mass calculation
It determines the number of atoms
It changes the chemical formula
Similar Resources on Wayground

6 questions
Manganese(II) Nitrate Molar Mass
Interactive video
•
9th - 10th Grade

7 questions
Molar Mass and Atomic Structure
Interactive video
•
9th - 10th Grade

6 questions
Molar Mass and Chemical Composition
Interactive video
•
9th - 10th Grade

6 questions
Molar Mass and Atomic Composition of KNO3
Interactive video
•
9th - 10th Grade

6 questions
Molar Mass and Atomic Structure
Interactive video
•
9th - 10th Grade

6 questions
Molecular Weight and Composition Questions
Interactive video
•
9th - 10th Grade

6 questions
Calculating Molar Mass of HBr
Interactive video
•
9th - 10th Grade

6 questions
Molar Mass and Chemical Composition
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
Molecules, Compounds, & Elements
Quiz
•
9th Grade