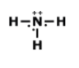
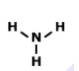
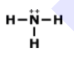
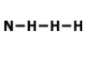Drawing Lewis Dot Structures
Quiz
•
Science
•
10th Grade
•
Practice Problem
•
Hard
Standards-aligned

Lisa Thompson
Used 1+ times
FREE Resource
Enhance your content in a minute
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
How many electrons should Nitrogen have around its Lewis dot model?
1
3
4
5
2.
REORDER QUESTION
1 min • 1 pt
Reorder the following steps for drawing lewis structures
If any atoms dont have an Octet, use electrons to create double bonds.
Add remaining electrons as Lone pairs (terminal atoms first).
Check each atom has the same number of electrons as it started with (formal charge)
The Valence electrons
charge - add/subtract if there is a charge
Place the lower Electronegative atom in the centre and draw in bonds
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Which of the following is the correct Lewis structure for the compound PBr3?
structure A
structure B
structure C
structure D
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
4.
DRAW QUESTION
1 min • 1 pt
Draw the lewis structure for CH3NH2

Tags
NGSS.HS-PS1-1
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

This could be the dot diagram of
Mg
Cl
C
O
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
6.
MULTIPLE CHOICE QUESTION
1 min • 12 pts
Each line in a Lewis Structure represents __________ electron(s).
3
2
1
4
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which Lewis Dot Model drawing correctly shows an O2 molecule?




Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

12 questions
Chapter Review EM Waves
Quiz
•
10th Grade

10 questions
Digestion and Excretion
Quiz
•
10th Grade

16 questions
QUÍMICA AGOSTO 2023
Quiz
•
10th Grade

10 questions
Nutrition in Plants and Animals
Quiz
•
7th - 10th Grade

10 questions
Extraneous variables
Quiz
•
10th - 12th Grade

10 questions
Slow loris for sakarat
Quiz
•
9th - 12th Grade

16 questions
Newspaper
Quiz
•
5th Grade - Professio...

10 questions
2. Life processes in living organisms part 1.10thScience and
Quiz
•
10th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Science

10 questions
Exploring the Layers of the Earth
Interactive video
•
6th - 10th Grade

10 questions
Exploring Weathering, Erosion, and Deposition Processes
Interactive video
•
6th - 10th Grade

13 questions
Part 3 Review Protein Synthesis and Mutations
Quiz
•
10th Grade

22 questions
Earth's Spheres and Interactions Quiz Reveiw
Quiz
•
8th - 10th Grade

35 questions
DNA Structure and Replication
Quiz
•
10th Grade

20 questions
Phylogenetic Trees and Cladograms
Quiz
•
10th Grade

89 questions
Unit 1 (Ch 2 & 3) Test Review - Water/Ocean Currents
Quiz
•
9th - 12th Grade

10 questions
Natural Resources
Lesson
•
9th - 12th Grade