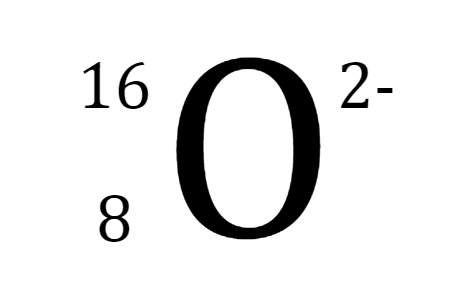Chemical Bonding
Quiz
•
Chemistry
•
9th Grade
•
Medium
Standards-aligned
Crissie Molskness
Used 6K+ times
FREE Resource
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Typically, atoms are more stable when they are
bonded together
apart from each other
Tags
NGSS.HS-PS1-4
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Why do atoms bond?
They typically don't bond
To add or take away energy levels
To have a full valance shell.
To have a full inner shell
Tags
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Ionic bonding is between a
nonmetal and nonmetal
metal and nonmetal
metal and metal
Depends on the situation
Tags
NGSS.HS-PS1-2
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Covalent bonding is between a
nonmetal and nonmetal
metal and nonmetal
metal and metal
It depends on the situation
Tags
NGSS.HS-PS1-2
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

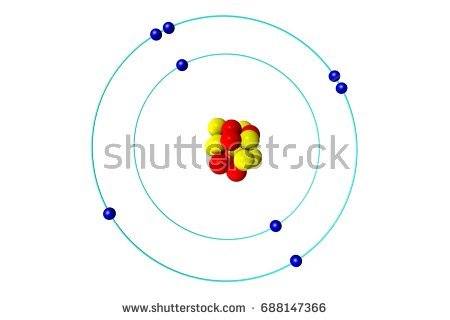
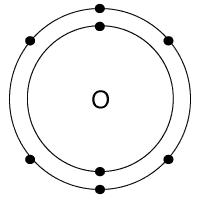
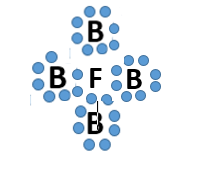
Which bond does this picture best represent?
Metallic bond
ionic bond
covalent bond
James Bond
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What do positive ions tend to do?
lose electrons
gain electrons
lose protons
gain protons
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What happens when magnesium loses 2 electrons?
It stabilizes to a net charge of 0
It turns into an atom
It becomes negatively charged
It becomes positively charged
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

15 questions
Chemical Bonds
Quiz
•
9th Grade

15 questions
Bonding
Quiz
•
9th Grade

20 questions
Chemical Bonding
Quiz
•
9th - 12th Grade

20 questions
Covalent Bonding with Names and Formulas
Quiz
•
8th - 10th Grade

17 questions
Bonding ionic covalent
Quiz
•
2nd - 12th Grade

15 questions
Ionic/covalent bonds
Quiz
•
9th - 12th Grade

15 questions
Chemistry Bonding
Quiz
•
9th Grade

15 questions
Chemical Bonding
Quiz
•
9th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Electron Configuration & Orbital Notation
Quiz
•
9th - 12th Grade

11 questions
Balancing Chemical Equations
Lesson
•
9th Grade

17 questions
Valence Electrons
Lesson
•
8th - 10th Grade

10 questions
Balancing Chemical Equations Challenge
Interactive video
•
6th - 10th Grade

22 questions
PHYSICAL AND CHEMICAL PROPERTIES
Quiz
•
7th - 9th Grade

18 questions
Ions
Quiz
•
9th - 12th Grade

30 questions
ERHS Chem Chapter 2 - The Atom
Quiz
•
9th - 12th Grade

20 questions
6_Periodic Table
Quiz
•
9th - 10th Grade