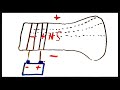
JJ Thompson's CRT Experiment Quiz
Interactive Video
•
Physics
•
9th - 10th Grade
•
Hard

Jennifer Brown
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What was the main significance of JJ Thompson's CRT experiment?
It demonstrated the wave nature of light.
It confirmed the atom as the smallest particle.
It led to the discovery of the electron.
It proved the existence of protons.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the main components of a cathode ray tube?
A wooden tube with a cathode
A solid metal tube with electrodes
A hollow glass tube with a cathode and anode
A plastic tube with a single electrode
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of the fluorescent material in a cathode ray tube?
To emit light when struck by cathode rays
To absorb the cathode rays
To deflect the cathode rays
To generate cathode rays
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is NOT a property of cathode rays as concluded by JJ Thompson?
They are streams of particles.
They travel in straight lines.
They carry a negative charge.
They are dependent on cathode composition.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How did JJ Thompson modify the cathode ray tube to discover the electron?
By increasing the temperature of the cathode
By changing the shape of the tube
By applying electric and magnetic fields
By using a different gas inside the tube
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What was the purpose of applying electric and magnetic fields in Thompson's experiment?
To heat the cathode
To deflect the cathode ray beam
To increase the speed of cathode rays
To change the color of the cathode rays
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What did the charge-to-mass ratio calculated by JJ Thompson indicate about the particles in cathode rays?
They had no mass.
They were about 2,000 times lighter than hydrogen atoms.
They were the same mass as hydrogen atoms.
They were heavier than hydrogen atoms.
Create a free account and access millions of resources
Similar Resources on Wayground

8 questions
Electric Field
Interactive video
•
9th - 10th Grade

11 questions
Particle Accelerators: From Giants to Miniatures
Interactive video
•
9th - 10th Grade

2 questions
Electric Field
Interactive video
•
9th - 10th Grade

6 questions
Cosmic Rays and Particle Detection Quiz
Interactive video
•
9th - 10th Grade

11 questions
Electrolysis Concepts and Reactions
Interactive video
•
9th - 10th Grade

11 questions
History of Radioactivity and Marie Curie
Interactive video
•
9th - 12th Grade

11 questions
Electromagnetic Radiation Concepts
Interactive video
•
9th - 10th Grade

11 questions
Properties and Uses of Electromagnetic Waves
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Physics

20 questions
Position vs. Time Graphs
Quiz
•
9th Grade

6 questions
Distance and Displacement
Lesson
•
10th - 12th Grade

15 questions
Position vs. Time and Velocity vs. Time Graphs
Quiz
•
10th - 12th Grade

20 questions
Specific heat capacity
Quiz
•
7th - 12th Grade

5 questions
Reading Motion Graphs
Lesson
•
8th - 10th Grade

9 questions
Position Vs. Time Graphs
Quiz
•
9th - 12th Grade

10 questions
Exit Check 2.4 - 2nd Law Graphs
Quiz
•
9th Grade

10 questions
Exit Check 2.5 - Earth's Layers
Quiz
•
9th Grade