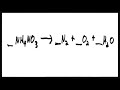
Balancing Chemical Equations Quiz
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard

Nancy Jackson
FREE Resource
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in balancing a chemical equation?
Add random coefficients to balance the equation.
Balance oxygen first.
Start with the element that appears most frequently.
Assign coefficients of zero to all species.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why should you avoid starting with oxygen when balancing this equation?
Oxygen is not present in the reactants.
Oxygen appears twice on the product side.
Oxygen is a noble gas.
Oxygen is already balanced.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you determine the coefficient for hydrogen in the equation?
By ensuring the number of hydrogen atoms is equal on both sides.
By doubling the number of hydrogen atoms on the reactant side.
By halving the number of hydrogen atoms on the product side.
By adding one more hydrogen atom to the reactant side.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of multiplying all coefficients by the denominator?
To decrease the number of products.
To increase the number of reactants.
To ensure the equation is balanced chemically.
To simplify the equation.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final step in balancing the chemical equation?
Add more reactants to the equation.
Ensure the equation is balanced chemically by adjusting coefficients.
Check if all elements are balanced mathematically.
Remove excess products from the equation.
Similar Resources on Wayground

6 questions
Balancing Combustion Reactions in Chemistry
Interactive video
•
9th - 10th Grade

7 questions
Balancing Oxygen Reactions
Interactive video
•
9th - 10th Grade

6 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

6 questions
Balancing Chemical Equations and Chlorine
Interactive video
•
9th - 10th Grade

6 questions
Chemical Reactions and Decomposition
Interactive video
•
9th - 10th Grade

6 questions
Balancing Chemical Reactions
Interactive video
•
9th - 10th Grade

6 questions
Balancing Chemical Equations Practice
Interactive video
•
9th - 10th Grade

6 questions
Balancing Chemical Reactions with Hydrogen
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade