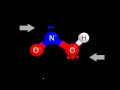
Polarity and Structure of HNO2
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Olivia Brooks
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in determining whether HNO2 is polar or nonpolar?
Checking the molecular weight
Analyzing the color of the compound
Examining the Lewis structure
Measuring the boiling point
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does the asymmetry in the Lewis structure of HNO2 suggest it might be polar?
Because asymmetry often leads to nonpolarity
Because asymmetry can create a separation of charge
Because it has a symmetrical distribution of electrons
Because it has a high molecular weight
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does electronegativity contribute to the polarity of HNO2?
It causes the molecule to become nonpolar
It makes the molecule symmetrical
It results in a positive and a negative side
It creates a uniform charge distribution
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom in HNO2 is more positive due to electronegativity differences?
Oxygen
All atoms are equally positive
Nitrogen
Hydrogen
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the molecular geometry of HNO2 reveal about its polarity?
It indicates a symmetrical structure
It suggests the molecule is neutral
It confirms the presence of poles
It shows that the molecule is nonpolar
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What color represents oxygen in the molecular model of HNO2?
Blue
White
Green
Red
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the electrostatic potential surface of HNO2 show?
A single uniform charge
Two distinct poles
No charge distribution
A neutral molecule
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the electrostatic potential model, what does the blue color indicate?
A positive charge
No charge
A neutral charge
A negative charge
Similar Resources on Wayground

11 questions
Ammonium Ion Properties and Behavior
Interactive video
•
9th - 10th Grade

11 questions
The Fascinating Properties of Water and Its Role in Life
Interactive video
•
9th - 10th Grade

11 questions
Molecular Polarity and Properties
Interactive video
•
9th - 12th Grade

11 questions
Understanding SO2: Structure and Polarity
Interactive video
•
9th - 10th Grade

11 questions
Solubility and Molecular Interactions
Interactive video
•
9th - 10th Grade

11 questions
Bonding and Molecular Geometry Concepts
Interactive video
•
9th - 10th Grade

7 questions
Nitrite Ion and Nitrous Acid Concepts
Interactive video
•
9th - 10th Grade

8 questions
Electronegativity and Compound Types
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
Molecules, Compounds, & Elements
Quiz
•
9th Grade