Electronegativity and Molecular Polarity
Interactive Video
•
Chemistry
•
10th - 12th Grade
•
Hard
Olivia Brooks
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
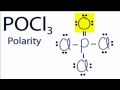
What is the primary reason the P3 molecule is not symmetrical?
It has an equal number of atoms on all sides.
It contains three chlorine atoms and one oxygen atom.
It is a linear molecule.
It has a central nitrogen atom.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does the oxygen atom in the P3 molecule have a slight negative charge?
Because it has a lower atomic number than chlorine.
Because it shares electrons equally with chlorine.
Because it is less electronegative than chlorine.
Because it is more electronegative than chlorine.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the result of having different electronegativities in the P3 molecule?
The molecule becomes symmetrical.
The molecule has distinct positive and negative poles.
The molecule forms a linear shape.
The molecule becomes non-polar.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following best describes the P3 molecule?
Polar due to equal distribution of electrons.
Non-polar due to equal electronegativity of atoms.
Polar due to asymmetrical structure and charge distribution.
Non-polar due to symmetrical structure.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of having poles in a molecule?
It indicates the molecule is non-polar.
It suggests the molecule is symmetrical.
It means the molecule has no charge distribution.
It confirms the molecule is polar.
Similar Resources on Wayground

8 questions
3.1 IMF's (Jeremy Krug)
Interactive video
•
11th Grade

10 questions
Molecular Structure and Dissociation Concepts
Interactive video
•
10th - 12th Grade

7 questions
Properties and Polarity of NH3
Interactive video
•
9th - 10th Grade

7 questions
Electronegativity and Molecular Polarity
Interactive video
•
9th - 10th Grade

8 questions
Polarity and Bonding in HCN
Interactive video
•
9th - 10th Grade

5 questions
Alkanes - Crash Course Organic Chemistry
Interactive video
•
11th Grade - University

6 questions
How Do Greenhouse Gases Actually Work?
Interactive video
•
11th Grade - University

11 questions
Valence Electrons and Formal Charges
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

14 questions
PERIODIC TRENDS
Quiz
•
11th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade