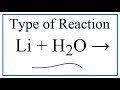
Lithium Reactions and Properties
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Ethan Morris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of reaction occurs when lithium reacts with water?
Single replacement reaction
Synthesis reaction
Double replacement reaction
Decomposition reaction
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the reaction between lithium and water, what does lithium replace?
Sodium
Chlorine
Hydrogen
Oxygen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the state of hydrogen when it is displaced in the reaction with lithium?
H
H2
H+
H-
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of lithium in the reaction with water?
It evaporates
It forms a precipitate
It acts as a catalyst
It displaces hydrogen
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for the compound formed when lithium reacts with water?
Li2O
LiO
LiOH
LiH
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What charge does lithium have in lithium hydroxide?
2+
1-
2-
1+
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final product when lithium reacts with water?
Lithium chloride
Lithium hydroxide
Lithium oxide
Lithium carbonate
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Balancing Chemical Equations and Solubility
Interactive video
•
9th - 10th Grade

7 questions
Lithium and Nitrite Compounds
Interactive video
•
9th - 10th Grade

11 questions
Mastering Ionic Equations in Chemical Reactions
Interactive video
•
9th - 10th Grade

9 questions
Lithium Chloride Properties and Behavior
Interactive video
•
9th - 10th Grade

9 questions
Acid-Base Reactions and Properties
Interactive video
•
9th - 10th Grade

11 questions
Lithium Reaction and Ionic Equations
Interactive video
•
9th - 10th Grade

9 questions
Balancing Chemical Equations with Polyatomic Ions
Interactive video
•
9th - 10th Grade

11 questions
Net Ionic Equations and Spectator Ions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
Molecules, Compounds, & Elements
Quiz
•
9th Grade