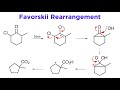
Favorskii Rearrangement Concepts
Interactive Video
•
Chemistry
•
11th - 12th Grade
•
Practice Problem
•
Hard
Liam Anderson
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Who invented the Favorskii rearrangement?
Dmitri Mendeleev
Alexei Favorskii
Linus Pauling
Marie Curie
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary application of the Favorskii rearrangement in synthesis?
Ring expansion
Ring contraction
Oxidation
Polymerization
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the Favorskii rearrangement, what intermediate is formed after the enolate attacks the carbon bearing the chloro group?
Cyclopentanone
Cyclobutanone
Cyclohexanone
Cyclopropanone
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the cyclopropanone intermediate in the Favorskii rearrangement?
It is stable and can be isolated
It forms a stable dimer
It is attacked by hydroxide base
It undergoes a Diels-Alder reaction
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a non-symmetrical system, how does the ring open in the Favorskii rearrangement?
To yield a radical
To yield a symmetrical carbanion
To yield the less substituted carbanion
To yield the more substituted carbanion
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a key feature of the Favorskii rearrangement mechanism?
It only works with symmetrical ketones
It yields the same carboxylate from isomeric alpha-haloketones
It requires a metal catalyst
It yields different carboxylates from isomeric alpha-haloketones
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens when anhydrous alkoxides are used as bases in the Favorskii rearrangement?
Alcohols are formed
Esters are formed
Aldehydes are formed
Carboxylate salts are formed
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

14 questions
PERIODIC TRENDS
Quiz
•
11th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade

18 questions
Naming and Formula Writing Ionic
Quiz
•
10th - 11th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade