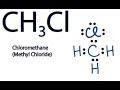
Valence Electrons and Lewis Structures
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Mia Campbell
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does Carbon have in the periodic table?
2
4
8
6
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is placed at the center of the CH3Cl Lewis structure?
Oxygen
Carbon
Chlorine
Hydrogen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to form bonds between the atoms in CH3Cl?
8
6
10
12
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in CH3Cl?
14
16
12
10
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Does each atom in CH3Cl have a full outer shell in the completed Lewis structure?
Yes, all atoms have full outer shells.
No, only Carbon and Chlorine have full outer shells.
No, only Hydrogen has a full outer shell.
No, none of the atoms have full outer shells.
Similar Resources on Wayground

6 questions
Boron and BH4- Lewis Structure
Interactive video
•
9th - 10th Grade

6 questions
Silicon Valence Electrons and Structures
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in CH2Cl2
Interactive video
•
9th - 10th Grade

6 questions
Lewis Structure of Methylamine
Interactive video
•
9th - 10th Grade

9 questions
Nitrogen and Its Ionic Forms
Interactive video
•
9th - 10th Grade

8 questions
Lewis Structure and Molecular Geometry of SBH3
Interactive video
•
9th - 10th Grade

9 questions
Understanding Lithium Ions and Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in Methanol
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
Molecules, Compounds, & Elements
Quiz
•
9th Grade