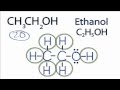
Valence Electrons and Lewis Structures
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is another name for the compound CH3CH2OH?
Methanol
Ethanol
Butanol
Propanol
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many total valence electrons are present in the Lewis structure of ethanol?
22
18
24
20
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of drawing lines between atoms in a Lewis structure?
To represent atomic mass
To show chemical bonds
To display molecular weight
To indicate electron spin
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to form chemical bonds in the initial skeleton structure of ethanol?
18
16
14
12
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the octet rule in the Lewis structure of ethanol?
It calculates the molecular weight
It ensures atoms have full outer shells
It predicts the boiling point
It determines the molecular shape
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does each hydrogen atom have in the ethanol Lewis structure?
Three
Four
Two
One
Similar Resources on Wayground

6 questions
Valence Electrons in CH2F2
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in CBr4
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Molecular Structure
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
Oxygen and Fluorine Chemistry Concepts
Interactive video
•
9th - 10th Grade

6 questions
NH2Cl Structure and Valence Electrons
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in PCl3
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Electron Configuration & Orbital Notation
Quiz
•
9th - 12th Grade