Formal Charges and Lewis Structures
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Emma Peterson
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are present in the CH4O Lewis structure?
18
16
14
12
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it unusual to see lone pairs on Carbon in Lewis structures?
Carbon is a noble gas.
Carbon prefers to have a positive charge.
Carbon usually forms four bonds.
Carbon is less electronegative than Oxygen.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of calculating formal charges in Lewis structures?
To calculate the molecular weight.
To identify the molecular geometry.
To find the most stable structure.
To determine the number of valence electrons.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
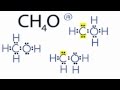
In the top structure, what are the formal charges on Carbon and Oxygen?
Carbon: +1, Oxygen: -1
Carbon: 0, Oxygen: 0
Carbon: +2, Oxygen: -1
Carbon: -1, Oxygen: +2
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which structure has formal charges closest to zero?
The structure with negative charge on Carbon.
The structure with positive charge on Oxygen.
The structure with negative charge on Oxygen and positive on Carbon.
The structure with no charges on any atoms.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important for formal charges to be close to zero?
It changes the molecular geometry.
It affects the color of the compound.
It increases the molecular weight.
It indicates a more stable structure.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the most appropriate Lewis structure for CH4O based on the video?
The structure with lone pairs on Carbon.
The structure with a negative charge on Carbon.
The structure with a positive charge on Oxygen.
The structure with formal charges closest to zero.
Similar Resources on Wayground

6 questions
CLEAN : The strongmans son Cambodias new politi
Interactive video
•
9th - 12th Grade

11 questions
Modern HTML and CSS from the Beginning (Including Sass) - CSS Variables (Custom Properties)
Interactive video
•
9th - 10th Grade

11 questions
Understanding Frequency and Data Analysis
Interactive video
•
9th - 10th Grade

11 questions
English Essentials - Powering Through Prose - 'Big Picture' Questions in Prose Fiction Analysis (Stage 4, Years/Grades 7
Interactive video
•
9th - 10th Grade

11 questions
Probability and Binomial Expressions
Interactive video
•
9th - 10th Grade

11 questions
Understanding Central Ideas and Secondary Headaches
Interactive video
•
9th - 10th Grade

11 questions
NASA's Apollo 11 Quarantine Procedures
Interactive video
•
9th - 10th Grade

11 questions
Television Commercials and Their Techniques
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 4/5-Covalent Bonding/Nomenclature
Quiz
•
10th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
Ions
Quiz
•
10th Grade

25 questions
VSPER Shape Quiz
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

61 questions
KAP Chemistry Covalent Test Review
Quiz
•
10th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade