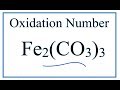
Oxidation Numbers and Carbonate Ions
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Aiden Montgomery
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of iron in Fe2(CO3)3?
+2
+3
+6
+4
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why must the oxidation numbers in a neutral compound add up to zero?
To balance the charges within the compound
To ensure the compound is stable
Because it is a rule for all compounds
To make the compound reactive
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of the carbonate ion (CO3)?
-1
+2
-2
-3
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do oxidation numbers relate to the charge of an ion?
They are always positive
They add up to the charge of the ion
They are always equal
They are always negative
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total oxidation number for three carbonate ions in Fe2(CO3)3?
-2
-8
-4
-6
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What must be the total positive oxidation number to balance the negative charge of the carbonate ions?
+2
+6
+4
+3
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the algebraic method, what does 'x' represent?
The total charge of the compound
The number of iron atoms
The charge of the carbonate ion
The oxidation number of iron
Create a free account and access millions of resources
Similar Resources on Wayground

8 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

10 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

9 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

11 questions
Oxidation Numbers and Ionic Compounds
Interactive video
•
9th - 10th Grade

11 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

7 questions
Understanding Carbonate Ion Properties
Interactive video
•
9th - 10th Grade

7 questions
Manganese and Carbonate Ions
Interactive video
•
9th - 10th Grade

11 questions
Net Ionic Equations and Spectator Ions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Electron Configuration & Orbital Notation
Quiz
•
9th - 12th Grade