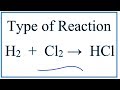
Chemical Reactions and Equations
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of reaction occurs when hydrogen gas reacts with chlorine gas to form hydrogen chloride?
Single displacement
Combination
Decomposition
Double displacement
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following best describes a synthesis reaction?
Two or more reactants combine to form a single product.
A single compound breaks down into two or more products.
An element replaces another in a compound.
Ions are exchanged between two compounds.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the state of hydrogen chloride formed in the reaction between hydrogen and chlorine gases?
Solid
Liquid
Gas
Plasma
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the reaction between hydrogen and chlorine considered exothermic?
It absorbs heat from the surroundings.
It releases a significant amount of heat.
It requires a catalyst to proceed.
It occurs only at high temperatures.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What coefficient is used to balance the hydrogen chloride in the equation H2 + Cl2 → HCl?
4
3
2
1
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a balanced chemical equation, what must be equal on both sides?
The total mass
The number of atoms for each element
The volume of gases
The number of molecules
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

21 questions
Isotopes and Ions
Quiz
•
9th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade