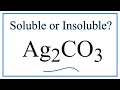
Solubility of Silver Carbonate
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Ethan Morris
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the solubility status of silver carbonate in water?
Highly soluble
Soluble
Insoluble
Partially soluble
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to solubility rules, which of the following is true about most carbonates?
They dissolve completely in water.
They are exceptions to solubility rules.
They are insoluble in water.
They are soluble in water.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ion combination is used to determine the solubility of silver carbonate using a solubility chart?
Ag+ and SO4 2-
Na+ and CO3 2-
Ag+ and CO3 2-
Ag+ and Cl-
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the 'i' symbol on the solubility chart indicate for silver carbonate?
It is soluble.
It is insoluble.
It is partially soluble.
It is highly soluble.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to silver carbonate when placed in water according to the solubility chart?
It dissociates into ions.
It remains intact and does not dissolve.
It dissolves completely.
It reacts with water.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the practical method mentioned to test the solubility of silver carbonate?
Using a pH meter
Placing it in water and observing
Mixing it with acid
Heating it in water
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the expected outcome when silver carbonate is placed in water based on the solubility rules and chart?
It will dissolve completely.
It will form a new compound.
It will remain mostly undissolved.
It will react with water.
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final conclusion about the solubility of Ag2CO3 in water?
Ag2CO3 is partially soluble in water.
Ag2CO3 is insoluble in water.
Ag2CO3 is soluble in water.
Ag2CO3 reacts with water.
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

21 questions
Isotopes and Ions
Quiz
•
9th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade