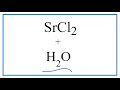
Dissolution of Strontium Chloride Concepts
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Emma Peterson
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the initial state of strontium chloride (SrCl2) when placed in water?
Gas
Solid
Plasma
Liquid
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which rule helps determine if strontium chloride will dissolve in water?
Periodic Table
Molecular Geometry
Electronegativity Chart
Solubility Rules
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of the strontium ion (Sr) when it dissociates in water?
2+
1-
1+
2-
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many chloride ions are formed when SrCl2 dissolves in water?
One
Two
Four
Three
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of water in the dissolution of SrCl2?
It acts as a solvent
It acts as a product
It acts as a reactant
It acts as a catalyst
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ion is represented as Cl- in the dissolution process?
Chloride ion
Oxygen ion
Hydrogen ion
Strontium ion
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the subscript '2' in SrCl2 indicate?
Two hydrogen ions
Two water molecules
Two strontium ions
Two chloride ions
Create a free account and access millions of resources
Similar Resources on Wayground

9 questions
Strontium Bromide and Water Reactions
Interactive video
•
9th - 10th Grade

9 questions
Strontium Hydrogen Carbonate Concepts
Interactive video
•
9th - 10th Grade

8 questions
Strontium Chlorite and Ionic Compounds
Interactive video
•
9th - 10th Grade

9 questions
Strontium Hydroxide Solubility Concepts
Interactive video
•
9th - 10th Grade

9 questions
Strontium Nitrate Chemistry Concepts
Interactive video
•
9th - 10th Grade

7 questions
Strontium and Ionic Compounds
Interactive video
•
9th - 10th Grade

6 questions
Health and Chemistry Concepts Assessment
Interactive video
•
9th - 10th Grade

7 questions
Understanding Strontium Chloride Compounds
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Electron Configuration & Orbital Notation
Quiz
•
9th - 12th Grade