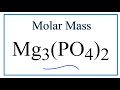
Molar Mass and Chemical Formulas
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Ethan Morris
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for magnesium phosphate?
Mg3(PO4)2
Mg2(PO4)3
Mg(PO4)2
Mg3(PO3)2
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many magnesium atoms are present in magnesium phosphate?
One
Two
Three
Four
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of magnesium used in the calculation?
16.00 grams per mole
40.08 grams per mole
30.97 grams per mole
24.31 grams per mole
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of phosphorus used in the calculation?
40.08 grams per mole
16.00 grams per mole
30.97 grams per mole
24.31 grams per mole
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many oxygen atoms are considered in the calculation for magnesium phosphate?
Two
Four
Six
Eight
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total molar mass of magnesium phosphate?
150.32 grams per mole
300.00 grams per mole
262.88 grams per mole
200.50 grams per mole
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why might there be slight variations in the calculated molar mass?
Incorrect multiplication
Different periodic tables have different values
Calculation errors
Different chemical formulas
Similar Resources on Wayground

11 questions
Understanding Density
Interactive video
•
9th - 12th Grade

6 questions
AP Physics 1 MCQ
Interactive video
•
9th - 12th Grade

8 questions
Thales of Miletus: Contributions and Beliefs
Interactive video
•
7th - 10th Grade

11 questions
Covalent Bonding Concepts and Diagrams
Interactive video
•
7th - 10th Grade

10 questions
Newton's Laws of Motion and Satellites
Interactive video
•
7th - 10th Grade

7 questions
#6-10 Front Page
Interactive video
•
9th Grade

6 questions
Understanding Black Holes and Escape Velocity
Interactive video
•
7th - 12th Grade

11 questions
Understanding Aging, Health, and Exercise
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

21 questions
Isotopes and Ions
Quiz
•
9th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade