What are lone pairs sometimes referred to as?
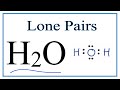
Lone Pairs and Water Geometry
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Shared pairs
Unbonded pairs
Paired electrons
Bonded pairs
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the Lewis structure of water, how many lone pairs are present?
Four
Two
Three
One
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of lone pairs in the molecular geometry of water?
They form new bonds
They have no effect
They increase the bond length
They influence the shape
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of water?
Tetrahedral
Trigonal planar
Linear
Bent
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do lone pairs affect the bond angles in water?
They increase the bond angles
They have no effect
They decrease the bond angles
They make the angles 180 degrees
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the approximate bond angle in water due to lone pairs?
180 degrees
105 degrees
109.5 degrees
120 degrees
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the molecular geometry of water when lone pairs are hidden?
It appears tetrahedral
It appears bent
It appears trigonal
It appears linear
Create a free account and access millions of resources
Similar Resources on Wayground

9 questions
Molecular Geometry and Electron Pairs
Interactive video
•
9th - 10th Grade

8 questions
Molecular Geometry and Bonding in HCN
Interactive video
•
9th - 10th Grade

8 questions
Molecular Geometry of Carbon Monoxide
Interactive video
•
9th - 10th Grade

8 questions
Molecular Geometry of HF
Interactive video
•
9th - 10th Grade

9 questions
Molecular Geometry and VSEPR Theory
Interactive video
•
9th - 10th Grade

10 questions
Xenon Tetrafluoride Geometry and Bonds
Interactive video
•
9th - 10th Grade

10 questions
Xenon Fluoride Molecular Geometry Concepts
Interactive video
•
9th - 10th Grade

10 questions
Lewis Structure and Molecular Geometry of OF2
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

25 questions
Equations of Circles
Quiz
•
10th - 11th Grade

30 questions
Week 5 Memory Builder 1 (Multiplication and Division Facts)
Quiz
•
9th Grade

33 questions
Unit 3 Summative - Summer School: Immune System
Quiz
•
10th Grade

10 questions
Writing and Identifying Ratios Practice
Quiz
•
5th - 6th Grade

36 questions
Prime and Composite Numbers
Quiz
•
5th Grade

14 questions
Exterior and Interior angles of Polygons
Quiz
•
8th Grade

37 questions
Camp Re-cap Week 1 (no regression)
Quiz
•
9th - 12th Grade

46 questions
Biology Semester 1 Review
Quiz
•
10th Grade
Discover more resources for Chemistry

25 questions
Equations of Circles
Quiz
•
10th - 11th Grade

30 questions
Week 5 Memory Builder 1 (Multiplication and Division Facts)
Quiz
•
9th Grade

33 questions
Unit 3 Summative - Summer School: Immune System
Quiz
•
10th Grade

37 questions
Camp Re-cap Week 1 (no regression)
Quiz
•
9th - 12th Grade

46 questions
Biology Semester 1 Review
Quiz
•
10th Grade