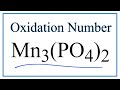
Oxidation States and Manganese Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the overall charge of the compound Mn3(PO4)2?
Neutral
Positive
Depends on the environment
Negative
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ion is present in Mn3(PO4)2 that helps determine the oxidation numbers?
Sulfate ion
Phosphate ion
Nitrate ion
Carbonate ion
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total oxidation number for the phosphate ion in Mn3(PO4)2?
-3
+6
-6
+3
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many manganese atoms are present in Mn3(PO4)2?
Four
One
Two
Three
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of each manganese atom in Mn3(PO4)2?
+1
+4
+2
+3
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If the phosphate ion contributes a -6 charge, what must the manganese atoms contribute to balance the compound?
+3
+6
+9
+12
Similar Resources on Wayground

8 questions
Oxidation Numbers and Nitrate Ions
Interactive video
•
9th - 10th Grade

8 questions
Understanding Manganese Compounds and Ions
Interactive video
•
9th - 10th Grade

9 questions
Oxidation Numbers in Phosphate Ion
Interactive video
•
9th - 10th Grade

6 questions
Oxidation Numbers and Ion Charges
Interactive video
•
9th - 10th Grade

7 questions
Understanding Oxidation Numbers in Chemistry
Interactive video
•
9th - 10th Grade

8 questions
Manganese and Carbonate Compounds
Interactive video
•
9th - 10th Grade

10 questions
Phosphate Ions and Iron Compounds
Interactive video
•
9th - 10th Grade

10 questions
Oxidation Numbers and Nickel Compounds
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

11 questions
Hallway & Bathroom Expectations
Quiz
•
6th - 8th Grade

20 questions
PBIS-HGMS
Quiz
•
6th - 8th Grade

10 questions
"LAST STOP ON MARKET STREET" Vocabulary Quiz
Quiz
•
3rd Grade

19 questions
Fractions to Decimals and Decimals to Fractions
Quiz
•
6th Grade

16 questions
Logic and Venn Diagrams
Quiz
•
12th Grade

15 questions
Compare and Order Decimals
Quiz
•
4th - 5th Grade

20 questions
Simplifying Fractions
Quiz
•
6th Grade

20 questions
Multiplication facts 1-12
Quiz
•
2nd - 3rd Grade