Bond Angles and Lone Pairs
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Emma Peterson
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
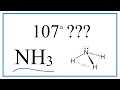
Why does NH3 have a bond angle of 107 degrees instead of 109.5 degrees?
Due to the presence of double bonds.
Because of the presence of a lone pair that is more repulsive than bonded atoms.
Because NH3 is a linear molecule.
Due to the presence of hydrogen bonds.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of NH3 due to the lone pair?
Trigonal planar
Linear
Tetrahedral
Trigonal pyramidal
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do lone pairs affect the bond angles in a molecule?
They increase the bond angles.
They have no effect on bond angles.
They decrease the bond angles due to their repulsive nature.
They make the molecule linear.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bond angle in water (H2O) and why?
180 degrees because it is a linear molecule.
109.5 degrees due to no lone pairs.
104.5 degrees due to two lone pairs pushing down.
120 degrees due to resonance.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the effect of two lone pairs on the bond angle in a molecule?
They make the molecule linear.
They have no effect on the bond angle.
They decrease the bond angle more than one lone pair.
They increase the bond angle.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bond angle in methane (CH4) and why?
107 degrees due to one lone pair.
104.5 degrees due to two lone pairs.
109.5 degrees because there are no lone pairs.
120 degrees due to resonance.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which molecule has the largest bond angle among NH3, H2O, and CH4?
NH3
H2O
CH4
All have the same bond angle
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following best describes the bond angle trend in molecules with lone pairs?
More lone pairs result in smaller bond angles.
Lone pairs make the bond angles equal to 180 degrees.
Lone pairs do not affect bond angles.
More lone pairs result in larger bond angles.
Similar Resources on Wayground

8 questions
Electron Geometry of Carbon Monoxide
Interactive video
•
9th - 10th Grade

10 questions
Hydroxide Ion Geometry Concepts
Interactive video
•
9th - 10th Grade

10 questions
Molecular Geometry of CH4
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry and Bonding in BrF5
Interactive video
•
9th - 10th Grade

11 questions
Bromine and Fluorine Bonding Concepts
Interactive video
•
9th - 10th Grade

11 questions
IF5 Molecular Geometry and Bonding
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry and AXN Notation
Interactive video
•
9th - 10th Grade

8 questions
Molecular Geometry of XeO3
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
Molecules, Compounds, & Elements
Quiz
•
9th Grade