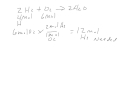
Limiting Reactants in Chemical Reactions
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of a limiting reactant in a chemical reaction?
It is always present in excess.
It determines the amount of product formed.
It has no effect on the reaction.
It speeds up the reaction.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the grilled cheese sandwich analogy, what was the limiting reactant?
Cheese
Butter
Tomato
Bread
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in solving a limiting reactant problem?
Determine the excess reactant.
Calculate the reaction rate.
Identify the products.
Write a balanced chemical equation.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the reaction 2 H2 + O2 → 2 H2O, which reactant is limiting if you have 4 moles of H2 and 6 moles of O2?
None
O2
H2
H2O
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many moles of H2O are formed from 4 moles of H2 in the reaction with O2?
4 moles
6 moles
8 moles
2 moles
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a mass-mass problem, what is the first step to determine the limiting reactant?
Calculate the molar mass of the reactants.
Write the balanced chemical equation.
Determine the volume of the reactants.
Identify the products.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If 25 g of iron oxide can produce 19.4 g of iron, what is the limiting reactant?
Iron oxide
Aluminum
Oxygen
Iron
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Limiting Reactants and Percent Yield
Interactive video
•
9th - 10th Grade

11 questions
Stoichiometry Concepts and Calculations
Interactive video
•
9th - 10th Grade

11 questions
Stoichiometry Concepts and Calculations
Interactive video
•
9th - 10th Grade

11 questions
Theoretical Yield and Gas Laws
Interactive video
•
9th - 10th Grade

11 questions
Stoichiometry and Dimensional Analysis
Interactive video
•
9th - 10th Grade

11 questions
Grams to Grams Stoichiometry in Chemical Reactions
Interactive video
•
9th - 10th Grade

11 questions
Limiting Reactants in Chemical Reactions
Interactive video
•
9th - 10th Grade

11 questions
Stoichiometry and Molar Calculations
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
Molecules, Compounds, & Elements
Quiz
•
9th Grade