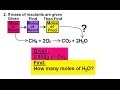
Moles and Reactions of Methane
Interactive Video
•
Chemistry, Science, Mathematics
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of reaction occurs when methane is burned in the presence of oxygen?
Synthesis reaction
Single replacement reaction
Decomposition reaction
Combustion reaction
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in determining the moles of water produced from methane?
Convert the mass of methane to moles
Measure the temperature change
Calculate the volume of methane
Determine the pressure of the system
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many hydrogen atoms are present in one molecule of methane?
Three
Four
One
Two
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of methane (CH4)?
20.00 g/mol
18.02 g/mol
16.04 g/mol
12.01 g/mol
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the ratio of moles of water produced to moles of methane used in the reaction?
2:1
3:1
1:2
1:1
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If you start with 0.0425 moles of methane, how many moles of water will be produced?
0.02125 moles
0.0425 moles
0.0850 moles
0.1700 moles
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final step in calculating the moles of water produced?
Convert moles of water to grams
Subtract moles of methane from water
Multiply moles of methane by the reaction ratio
Add moles of methane and water
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Understanding Limiting and Excess Reactants
Interactive video
•
9th - 10th Grade

11 questions
Stoichiometry and Energy Calculations
Interactive video
•
9th - 10th Grade

10 questions
Balancing Reactions of Methane and Chlorine
Interactive video
•
9th - 10th Grade

6 questions
Limiting Reactants and Product Formation
Interactive video
•
9th - 10th Grade

11 questions
Moles to Particles Conversion in Chemistry
Interactive video
•
9th - 10th Grade

11 questions
Stoichiometry and Chemical Reactions
Interactive video
•
9th - 10th Grade

11 questions
Chemical Yield and Molar Calculations
Interactive video
•
9th - 10th Grade

11 questions
Haber Process and Reaction Dynamics
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
Molecules, Compounds, & Elements
Quiz
•
9th Grade