What is the main focus of the video tutorial?
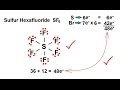
Sulfur Hexafluoride Bonding Concepts
Interactive Video
•
Chemistry, Science, Biology
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
The octet rule in chemistry
Molecules that do not follow the octet rule
The structure of water molecules
The periodic table of elements
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does sulfur have?
Seven
Four
Six
Eight
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is fluorine eager to form a single bond?
To gain an electron
To share electrons
To lose electrons
To gain stability
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of sulfur in the sulfur hexafluoride molecule?
It remains unbonded
It acts as a central atom forming six bonds
It donates electrons to fluorine
It forms double bonds with fluorine
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many bonds does sulfur form in sulfur hexafluoride?
Four
Seven
Five
Six
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the electron arrangement in each fluorine atom in sulfur hexafluoride?
A sextet
A doublet
A triplet
An octet
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the term used to describe sulfur's electron arrangement in sulfur hexafluoride?
Standard octet
Expanded octet
Incomplete octet
Double octet
Create a free account and access millions of resources
Similar Resources on Quizizz

9 questions
Valence Electrons and Formal Charge
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

9 questions
Lewis Structures and Valence Electrons
Interactive video
•
9th - 10th Grade

11 questions
Electron Configurations and Atomic Structure
Interactive video
•
9th - 10th Grade

9 questions
Valence Electrons and Octet Rule
Interactive video
•
9th - 10th Grade

10 questions
Barium and Sulfur Compounds
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Molecular Structure
Interactive video
•
9th - 10th Grade

10 questions
HXeSH Lewis Structure and Valence Electrons
Interactive video
•
9th - 10th Grade
Popular Resources on Quizizz

15 questions
Multiplication Facts
Quiz
•
4th Grade

20 questions
Math Review - Grade 6
Quiz
•
6th Grade

20 questions
math review
Quiz
•
4th Grade

5 questions
capitalization in sentences
Quiz
•
5th - 8th Grade

10 questions
Juneteenth History and Significance
Interactive video
•
5th - 8th Grade

15 questions
Adding and Subtracting Fractions
Quiz
•
5th Grade

10 questions
R2H Day One Internship Expectation Review Guidelines
Quiz
•
Professional Development

12 questions
Dividing Fractions
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Spanish preterite verbs (irregular/changed)
Quiz
•
9th - 10th Grade

10 questions
Identify Slope and y-intercept (from equation)
Quiz
•
8th - 9th Grade

10 questions
Juneteenth: History and Significance
Interactive video
•
7th - 12th Grade

8 questions
"Keeping the City of Venice Afloat" - STAAR Bootcamp, Day 1
Quiz
•
9th - 12th Grade

26 questions
June 19th
Quiz
•
4th - 9th Grade

20 questions
Distance, Midpoint, and Slope
Quiz
•
10th Grade

20 questions
Figurative Language Review
Quiz
•
10th Grade

27 questions
STAAR English 1 Review
Quiz
•
9th Grade