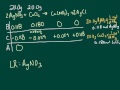
Stoichiometry Concepts and Calculations
Interactive Video
•
Chemistry, Science, Mathematics
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary objective of using a BCA table in stoichiometry?
To find the theoretical amounts of products and excess reactants
To determine the speed of a reaction
To calculate the pH of a solution
To measure the temperature change in a reaction
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the given problem, which two compounds are initially reacted?
Silver nitrate and sodium chloride
Calcium nitrate and silver chloride
Silver nitrate and calcium chloride
Calcium chloride and sodium nitrate
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you convert grams to moles in stoichiometry?
By subtracting the molar mass from the grams
By adding the grams to the molar mass
By multiplying the grams by the molar mass
By dividing the grams by the molar mass
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the term 'limiting reactant' refer to?
The reactant that changes color during the reaction
The reactant that speeds up the reaction
The reactant that determines the amount of product formed
The reactant that is left over after the reaction
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the limiting reactant determined in a chemical reaction?
By comparing the initial masses of reactants
By comparing the mole ratios of reactants
By observing the color change
By measuring the temperature change
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the excess reactant in a reaction?
It speeds up the reaction
It is completely used up in the reaction
It remains after the reaction is complete
It changes the color of the solution
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you convert moles of a substance to grams?
Multiply the moles by the molar mass
Divide the moles by the molar mass
Add the moles to the molar mass
Subtract the molar mass from the moles
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Stoichiometry and Chemical Reactions
Interactive video
•
9th - 12th Grade

11 questions
Limiting Reactants and Chemical Reactions
Interactive video
•
9th - 12th Grade

11 questions
Limiting Reactants and Chemical Formulas
Interactive video
•
9th - 12th Grade

11 questions
Limiting Reactants and Molar Mass
Interactive video
•
10th - 12th Grade

11 questions
Stoichiometry and Acid-Base Reactions
Interactive video
•
9th - 12th Grade

11 questions
Chemical Reactions and Yield Calculations
Interactive video
•
9th - 12th Grade

11 questions
Neutralization Reactions and Calculations
Interactive video
•
10th - 12th Grade

11 questions
Limiting Reactants and Stoichiometry
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

20 questions
Naming Covalent Compounds
Quiz
•
11th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade