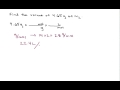
Unit Conversion and Molecular Mass
Interactive Video
•
Chemistry, Science, Mathematics
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why can't you directly convert grams to volume?
Because grams are only used for solids.
Because you need to use moles as an intermediary step.
Because volume is not a measurable unit.
Because grams and volume are unrelated units.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in converting grams to volume?
Convert liters to moles.
Convert grams to moles.
Convert grams to liters directly.
Convert moles to grams.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the relationship between grams and moles?
Grams are always larger than moles.
Moles are used to measure volume.
Grams per mole is used to find molecular mass.
Moles are used to measure weight.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you calculate the molecular mass of N2?
Subtract the atomic mass of nitrogen from 28.
Multiply the atomic mass of nitrogen by three.
Divide the atomic mass of nitrogen by two.
Add the atomic masses of two nitrogen atoms.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the volume occupied by one mole of gas at STP?
28 liters
22.4 liters
44.8 liters
14 liters
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final step in the conversion process?
Multiply the result by 22.4 liters.
Add grams and moles together.
Divide moles by grams.
Multiply grams by liters.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final volume obtained in the example?
3.72 liters
22.4 liters
28 liters
4.65 liters
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Understanding Moles in Chemistry
Interactive video
•
9th - 10th Grade

9 questions
Volume Calculations for Gases
Interactive video
•
9th - 10th Grade

11 questions
Mole Concept and Conversions
Interactive video
•
9th - 10th Grade

8 questions
Molar Mass and Conversion Techniques
Interactive video
•
9th - 10th Grade

9 questions
Molar Mass and Conversion Factors
Interactive video
•
9th - 10th Grade

10 questions
Calculating Moles from Gas Volume
Interactive video
•
9th - 10th Grade

11 questions
Understanding Molarity and Solution Preparation
Interactive video
•
9th - 10th Grade

11 questions
Copper Sulfate Molarity and Properties
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
Molecules, Compounds, & Elements
Quiz
•
9th Grade