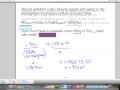
Combustion of Ethane and Stoichiometry
Interactive Video
•
Chemistry, Mathematics, Science
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary focus of stoichiometry in chemical reactions?
Predicting reaction products
Calculating energy changes
Balancing chemical equations
Understanding chemical properties
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the reaction of sulfur trioxide with water, what is produced along with sulfuric acid?
Oxygen
Heat
Carbon dioxide
Hydrogen gas
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How much energy is released when 1 mole of sulfur trioxide reacts with water?
29.6 KJ
943.73 KJ
129.6 KJ
80.62 KJ
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of sulfur trioxide used in the calculations?
32 g/mol
142.85 g/mol
80.62 g/mol
18 g/mol
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to consider significant figures in chemical calculations?
To ensure accuracy
To avoid errors
To make calculations faster
To simplify calculations
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in solving the combustion of ethane problem?
Calculating energy released
Balancing the equation
Writing the chemical equation
Calculating moles of water
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the products of ethane combustion?
Carbon dioxide and water
Carbon monoxide and water
Methane and water
Oxygen and hydrogen
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Conformations and Newman Projections
Interactive video
•
10th - 12th Grade

11 questions
Chemical Reactions and Their Impact on Life and Technology
Interactive video
•
9th - 10th Grade

11 questions
Understanding Hess's Law and Enthalpy Changes
Interactive video
•
9th - 12th Grade

11 questions
Chemical Processes in Heat Solutions
Interactive video
•
9th - 10th Grade

11 questions
Chemical Reactions and Energy Changes
Interactive video
•
9th - 10th Grade

11 questions
Stoichiometry and Enthalpy Concepts
Interactive video
•
9th - 10th Grade

11 questions
Understanding the Mole and Calculating Substances
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

20 questions
Naming Covalent Compounds
Quiz
•
11th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade