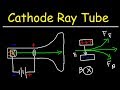
Cathode Ray Tube Experiment Quiz
Interactive Video
•
Physics, Science
•
9th - 12th Grade
•
Medium
Lucas Foster
Used 2+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What was the primary discovery made by J.J. Thompson using the cathode ray tube experiment?
The existence of protons
The presence of electrons in atoms
The structure of the nucleus
The concept of atomic mass
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to maintain a low pressure inside the cathode ray tube?
To increase the speed of the electrons
To prevent the tube from overheating
To ensure the electron beam is visible
To allow the electrons to collide with gas particles
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of the anode in the cathode ray tube?
To create a magnetic field
To heat the cathode
To emit electrons
To attract electrons
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the experiment called the 'cathode ray tube' experiment?
Because the anode emits the rays
Because the cathode is positively charged
Because the electron beam originates from the cathode
Because the tube is made of cathode material
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How did J.J. Thompson determine that the cathode ray consists of negatively charged particles?
By measuring the temperature of the cathode
By changing the pressure inside the tube
By using a magnet to deflect the beam
By observing the color of the glow
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What conclusion did Thompson draw from using different cathode materials?
The cathode material affects the speed of electrons
Cathode rays are unique to each metal
All atoms contain negatively charged particles
Only certain metals can emit cathode rays
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the radius of curvature in the cathode ray tube experiment?
It determines the color of the glow
It affects the pressure inside the tube
It helps calculate the charge-to-mass ratio
It changes the speed of the electrons
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

8 questions
Production of Electromagnetic Waves from Atoms and Nuclei
Interactive video
•
10th Grade - University

11 questions
X-ray Machine Components and Functions
Interactive video
•
11th Grade - University

6 questions
Our First Glimpse of the Dark Side of the Moon
Interactive video
•
11th Grade - University

11 questions
Atomic Models and Theories
Interactive video
•
9th - 12th Grade

11 questions
The Discovery and Impact of X-Rays
Interactive video
•
9th - 12th Grade

6 questions
Cathode ray
Interactive video
•
6th - 12th Grade

6 questions
¿Cómo surgieron los videojuegos? 12 datos curiosos
Interactive video
•
10th - 12th Grade

11 questions
JJ Thompson's CRT Experiment Quiz
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Physics

25 questions
Newton's Laws of Motion
Quiz
•
9th Grade

15 questions
Position vs. Time and Velocity vs. Time Graphs
Quiz
•
10th - 12th Grade

73 questions
S1 Interim Review Physics
Quiz
•
9th - 12th Grade

20 questions
Calculating Net Force
Quiz
•
6th - 9th Grade

18 questions
Net Forces
Quiz
•
9th Grade

20 questions
Position vs. Time Graphs
Quiz
•
9th Grade

37 questions
Forces-Conceptual Physics
Quiz
•
9th - 12th Grade

32 questions
Unit 3 EM Spectrum Quizizz Review - 2025/2026
Quiz
•
9th Grade