Chemical Reaction Kinetics Concepts
Interactive Video
•
Chemistry, Science
•
10th Grade - University
•
Practice Problem
•
Hard
Emma Peterson
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
For a zero-order reaction, what happens to the rate if the concentration of reactant A is doubled?
The rate doubles.
The rate triples.
The rate remains unchanged.
The rate quadruples.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the units of the rate constant k for a first-order reaction?
M to the first power times T to the negative one
M to the zero power times T to the negative one
M to the negative two times T to the negative one
M to the negative one times T to the negative one
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which statement is true about the half-life of a first-order reaction?
It depends on the initial concentration.
It is independent of the initial concentration.
It decreases with the initial concentration.
It increases with the initial concentration.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
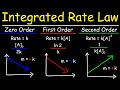
What is the integrated rate law expression for a first-order reaction?
Final concentration = -kt + initial concentration
ln(final concentration) = -kt + ln(initial concentration)
1/final concentration = kt + 1/initial concentration
Final concentration = kt + initial concentration
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
For a second-order reaction, what plot gives a straight line?
1/concentration vs. time
Rate vs. concentration
ln(concentration) vs. time
Concentration vs. time
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If a reaction is third order overall, what are the units of the rate constant k?
M to the first power times T to the negative one
M to the zero power times T to the negative one
M to the negative two times T to the negative one
M to the negative three times T to the negative one
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which plot corresponds to a zero-order reaction?
Concentration vs. time with a positive slope
Concentration vs. time with a negative slope
ln(concentration) vs. time with a negative slope
1/concentration vs. time with a positive slope
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 4/5-Covalent Bonding/Nomenclature
Quiz
•
10th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
Ions
Quiz
•
10th Grade

25 questions
VSPER Shape Quiz
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
PERIODIC TRENDS
Quiz
•
11th Grade

61 questions
KAP Chemistry Covalent Test Review
Quiz
•
10th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade