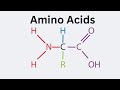
Understanding L Alpha Amino Acids
Interactive Video
•
Biology, Science, Chemistry
•
8th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the central element in L Alpha amino acids?
Alpha Carbon
Oxygen
Hydrogen
Nitrogen
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where is the amino group located in relation to the alpha carbon?
To the right of the alpha carbon
To the left of the alpha carbon
Above the alpha carbon
Below the alpha carbon
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What determines the chemical properties of an amino acid?
The amino group
The alpha carbon
The carboxyl group
The R Group or side chain
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of side chain is hydrophobic?
Polar side chain
Nonpolar side chain
Ionic side chain
Acidic side chain
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which elements are typically found in polar side chains?
Calcium and magnesium
Sulfur and phosphorus
Nitrogen and oxygen
Carbon and hydrogen
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of the R Group in amino acids?
It forms the backbone of the amino acid
It connects the amino group and carboxyl group
It determines the amino acid's chemical properties
It stabilizes the alpha carbon
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the motivational message at the end of the video?
Knowledge is power
Kindness multiplies kindness
Practice makes perfect
Stay curious
Similar Resources on Wayground

11 questions
Understanding Protein Structure and Function
Interactive video
•
9th - 10th Grade

11 questions
Photosynthesis and the Calvin Cycle Quiz
Interactive video
•
9th - 10th Grade

11 questions
Understanding Protein Structure
Interactive video
•
9th - 12th Grade

6 questions
Origins of Life and Asteroid Bennu
Interactive video
•
9th - 10th Grade

6 questions
Bacterial Interactions with Legume Plants
Interactive video
•
7th - 10th Grade

9 questions
Transport Mechanisms in Plants: Xylem and Phloem
Interactive video
•
9th Grade

6 questions
Understanding Chemical Reactions in Biology
Interactive video
•
9th - 10th Grade

11 questions
Protein Structure and Function Concepts
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Biology

20 questions
Cell Organelles
Quiz
•
9th Grade

20 questions
Cell organelles and functions
Quiz
•
10th Grade

20 questions
Cell Organelles
Quiz
•
9th Grade

16 questions
AP Biology: Unit 1 Review (CED)
Quiz
•
9th - 12th Grade

22 questions
Macromolecules
Quiz
•
9th Grade

15 questions
Enzymes
Quiz
•
9th Grade

20 questions
The Cell Cycle
Quiz
•
9th Grade

20 questions
Macromolecules
Quiz
•
10th Grade