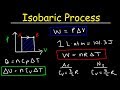
Thermodynamics and Gas Laws
Interactive Video
•
Physics, Chemistry, Science
•
10th - 12th Grade
•
Hard
Olivia Brooks
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formula used to calculate the work done by a gas during an isobaric process?
W = nRT
W = PΔV
W = PV
W = nCvΔT
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In an isobaric process, if a gas expands, what is the sign of the work done?
Positive
Undefined
Zero
Negative
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which law relates the volume and temperature of a gas at constant pressure?
Boyle's Law
Charles's Law
Avogadro's Law
Dalton's Law
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the value of the gas constant R when using liters and atm?
8.3145 J/mol·K
0.08206 L·atm/mol·K
1.013 L·atm/mol·K
22.4 L/mol
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does the temperature change when the volume of a gas doubles at constant pressure?
It triples
It doubles
It remains the same
It halves
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar heat capacity at constant pressure (Cp) for a monoatomic gas?
9/2 R
5/2 R
3/2 R
7/2 R
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following gases is considered monoatomic?
Carbon Dioxide
Helium
Nitrogen
Oxygen
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

6 questions
Gases in Liquids - Henrys Law
Interactive video
•
10th Grade - University

11 questions
Gas Laws and Molar Mass Calculations
Interactive video
•
10th - 12th Grade

11 questions
Thermodynamic Processes and Properties
Interactive video
•
11th - 12th Grade

11 questions
Internal Energy and Heat Capacity
Interactive video
•
10th - 12th Grade

10 questions
Density and Ideal Gas Law Concepts
Interactive video
•
10th - 12th Grade

8 questions
Ideal Gas Law
Interactive video
•
9th - 12th Grade

11 questions
Gas Law Concepts and Calculations
Interactive video
•
10th - 12th Grade

11 questions
Understanding the Combined Gas Law
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Physics

15 questions
Position vs. Time and Velocity vs. Time Graphs
Quiz
•
10th - 12th Grade

73 questions
S1 Interim Review Physics
Quiz
•
9th - 12th Grade

37 questions
Forces-Conceptual Physics
Quiz
•
9th - 12th Grade

20 questions
Newtons Laws of Motion
Quiz
•
10th - 11th Grade

18 questions
Conservation of Energy
Quiz
•
10th Grade

107 questions
Physics Interim Review Game
Quiz
•
11th Grade

46 questions
Acceleration and Force Equations
Quiz
•
11th Grade - University

25 questions
Newton's Second Law
Quiz
•
11th Grade