Exploring Types of Chemical Reactions
Interactive Video
•
Science
•
6th - 10th Grade
•
Easy
Aiden Montgomery
Used 10+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many types of chemical reactions are you responsible for knowing?
Four
Five
Three
Six
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the term 'synthesis' mean in the context of chemical reactions?
To make or build
To replace
To break down
To combust
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a synthesis reaction, what happens to the simpler materials?
They are broken down
They are replaced
They are combusted
They are combined to form a more complex compound
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the key characteristic of a decomposition reaction?
Combining simpler materials
Replacing one element with another
Breaking down a complex compound into simpler compounds
Combusting a fuel source
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a decomposition reaction, what happens to the complex compound?
It is broken down into simpler compounds
It replaces another compound
It is combined with another compound
It combusts
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
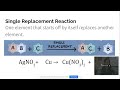
What occurs in a single replacement reaction?
One element replaces another in a compound
A complex compound breaks down
Two compounds switch elements
Two elements combine
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a single replacement reaction, what happens to the element that starts off by itself?
It combusts
It replaces another element in a compound
It breaks down
It combines with another element
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

11 questions
Identifying Types of Chemical Reactions with Crocs
Interactive video
•
6th - 10th Grade

11 questions
Understanding Chemical Formulas and Reactions
Interactive video
•
7th - 10th Grade

11 questions
Understanding Physical and Chemical Changes
Interactive video
•
6th - 10th Grade

8 questions
Activity Series and Single Replacement Reactions
Interactive video
•
8th - 10th Grade

11 questions
Exploring the Solubility Chart: Table F Insights
Interactive video
•
6th - 10th Grade

11 questions
Exploring Types of Chemical Reactions
Interactive video
•
6th - 10th Grade

11 questions
Identifying Types of Chemical Reactions
Interactive video
•
6th - 10th Grade

11 questions
Exploring the Order of the Periodic Table
Interactive video
•
6th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Science

20 questions
Physical and Chemical Changes
Quiz
•
8th Grade

22 questions
Newton's Laws of Motion
Lesson
•
8th Grade

20 questions
Distance Time Graphs
Quiz
•
6th - 8th Grade

26 questions
7.6E Rate of Dissolution
Quiz
•
7th Grade

21 questions
Balanced and Unbalanced Forces
Quiz
•
8th Grade

17 questions
Energy Transformations
Quiz
•
6th - 8th Grade

10 questions
Exploring Newton's Laws of Motion
Interactive video
•
6th - 10th Grade

17 questions
Thermal Energy Transfer
Lesson
•
6th - 8th Grade