Unit 2 Atomic Theory Part 2 Test Review
Quiz
•
Science
•
10th Grade
•
Hard
+1
Standards-aligned
Braegan Koen
Used 1+ times
FREE Resource
14 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
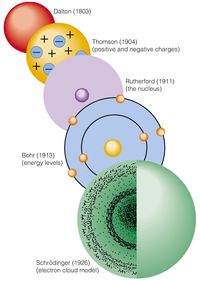
Which model of the atom consists of protons and neutrons in a central nucleus, with electrons occupying discrete energy levels surrounding the nucleus?
Rutherford Model
Planck Model
Bohr Model
Quantum Model
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Scientists are consistently building on each other’s models. What did Bohr add to the model of the atom?
That atoms are solid structures and simple building blocks.
That atoms have protons and electrons stuck together.
That atoms have orbiting electrons that can jump energy levels.
That there is an electron cloud around a nucleus.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

In the Bohr model of an atom, protons are represented in blue, the neutrons are red, and the electrons are green. Use your periodic table and determine which of the following atoms is represented by the Bohr model.
Magnesium
Calcium
Aluminum
Sodium
Tags
NGSS.HS-PS1-1
4.
MULTIPLE SELECT QUESTION
30 sec • 1 pt
A group of students was asked to generate statements about the structure of an atom. Which statements accurately describe the structure of atoms? Select TWO correct answers.
Valence electrons are the electrons located on the outermost energy level.
Electrons are neutrally charged and located outside the nucleus.
The nucleus of an atom contains positively charged protons and negatively charged electrons.
Protons are positively charged and located outside the nucleus.
The number of neutrons is equal to the mass number minus the number of protons.
Tags
NGSS.MS-PS1-1
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which area of a Bohr Model shows an electron occupying the lowest energy level outside the nucleus of an atom?
First shell
Second shell
Third shell
Fourth shell
Tags
NGSS.HS-PS1-1
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

Select which color of visible light has the lowest frequency, and thus, the lowest energy.
Red
Orange
Blue
Violet
Tags
NGSS.HS-PS4-1
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

Which of the following waves would have the lowest energy?
Red wave
Green wave
Blue wave
All three waves have the same energy
Tags
NGSS.HS-PS4-1
NGSS.HS-PS4-4
Create a free account and access millions of resources
Similar Resources on Wayground

15 questions
Models
Quiz
•
8th Grade - University

17 questions
Atoms & Periodic Table Vocabulary
Quiz
•
8th - 10th Grade

15 questions
Atomic Physics
Quiz
•
10th Grade - University

10 questions
STRUCTURE OF AN ATOM
Quiz
•
9th - 10th Grade

10 questions
Electrons
Quiz
•
10th - 12th Grade

15 questions
Atomic Bohr Models
Quiz
•
8th Grade - University

15 questions
Atomic Structure Vocabulary
Quiz
•
8th Grade - University

15 questions
Atomic Structure
Quiz
•
8th Grade - University
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Science

10 questions
Exploring Newton's Laws of Motion
Interactive video
•
6th - 10th Grade

10 questions
Exploring Chemical and Physical Changes
Interactive video
•
6th - 10th Grade

10 questions
Exploring the States of Matter
Interactive video
•
6th - 10th Grade

10 questions
Exploring the States of Matter and Thermal Energy
Interactive video
•
6th - 10th Grade

10 questions
Exploring Light and Waves Concepts
Interactive video
•
6th - 10th Grade

10 questions
Exploring Weathering, Erosion, and Deposition Processes
Interactive video
•
6th - 10th Grade

16 questions
Macromolecules Quiz
Quiz
•
10th Grade

24 questions
DNA Structure and Replication
Quiz
•
10th Grade
