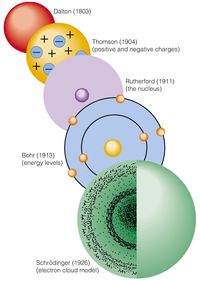
3.1 History of Chemistry
Quiz
•
Chemistry
•
10th Grade
•
Easy
+2
Standards-aligned
Conner Wyatt
Used 10+ times
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 10 pts
What is the name of the atomic model proposed by John Dalton?
Billiard Ball Model
Plum Pudding Model
Nuclear Model
Quantum Mechanical Model
2.
MULTIPLE CHOICE QUESTION
30 sec • 10 pts
Fill in the blank: In reactions, atoms are combined, separated, or ________, according to John Dalton.
rearranged
destroyed
multiplied
dissolved
Tags
NGSS.HS-PS1-7
3.
MULTIPLE CHOICE QUESTION
30 sec • 10 pts
Electrical fields and magnetic fields form ________ waves.
electromagnetic
mechanical
sound
thermal
Tags
NGSS.HS-PS2-5
NGSS.HS-PS4-3
NGSS.HS-PS4-5
4.
MULTIPLE CHOICE QUESTION
30 sec • 10 pts
Who demonstrated electromagnetic waves in 1887?
Heinrich Hertz
James Clerk Maxwell
Albert Einstein
Isaac Newton
5.
MULTIPLE CHOICE QUESTION
30 sec • 10 pts
The unit of frequency (a cycle per second) is named after whom?
Newton
Hertz
Tesla
Faraday
Tags
NGSS.HS-PS4-1
6.
MULTIPLE CHOICE QUESTION
30 sec • 10 pts
Who discovered the electron in 1904?
J. J. Thomson
Ernest Rutherford
Niels Bohr
Marie Curie
7.
MULTIPLE CHOICE QUESTION
30 sec • 10 pts
The Planetary Model works well for simple atoms but not for larger, more complex atoms.
True
False
Create a free account and access millions of resources
Similar Resources on Wayground

10 questions
Teori atom dan partikel penyusun atom
Quiz
•
10th Grade

10 questions
Atomic Theory History
Quiz
•
8th - 10th Grade

14 questions
Atomic Theory
Quiz
•
9th - 11th Grade

15 questions
Atomic Theory
Quiz
•
6th - 10th Grade

15 questions
Atomic Theory
Quiz
•
6th - 10th Grade

10 questions
Atomic Structure
Quiz
•
8th - 12th Grade

10 questions
Perkembangan Teori Atom
Quiz
•
10th Grade

10 questions
Quiz Perkembangan Teori Atom
Quiz
•
10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

24 questions
Virus Review
Quiz
•
10th Grade
