
Quantum Mechanical Model of the Atom
Quiz
•
Science
•
9th Grade
•
Practice Problem
•
Medium
Standards-aligned

Lisa Thompson
Used 1+ times
FREE Resource
Enhance your content in a minute
25 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which shape represents the shape of an "s" orbital?
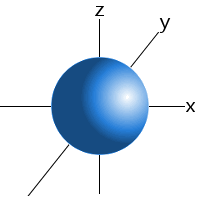
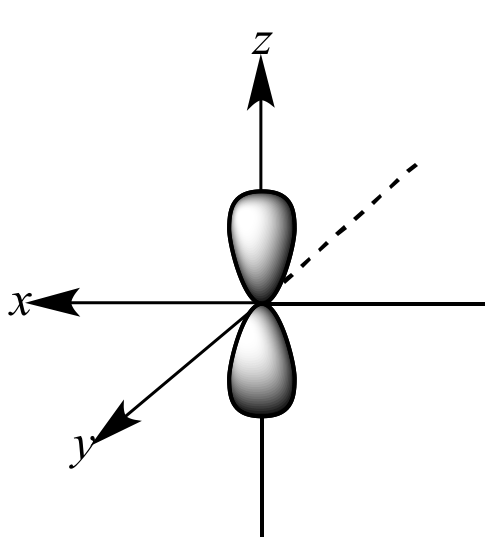
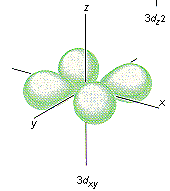
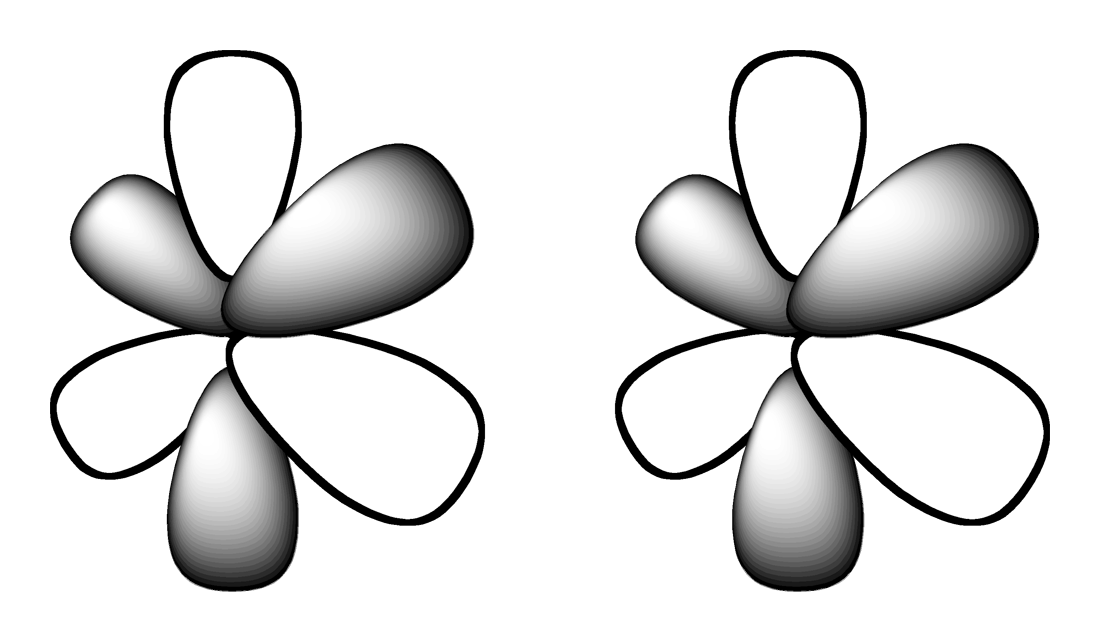
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which shape represents the shape of an "p" orbital?



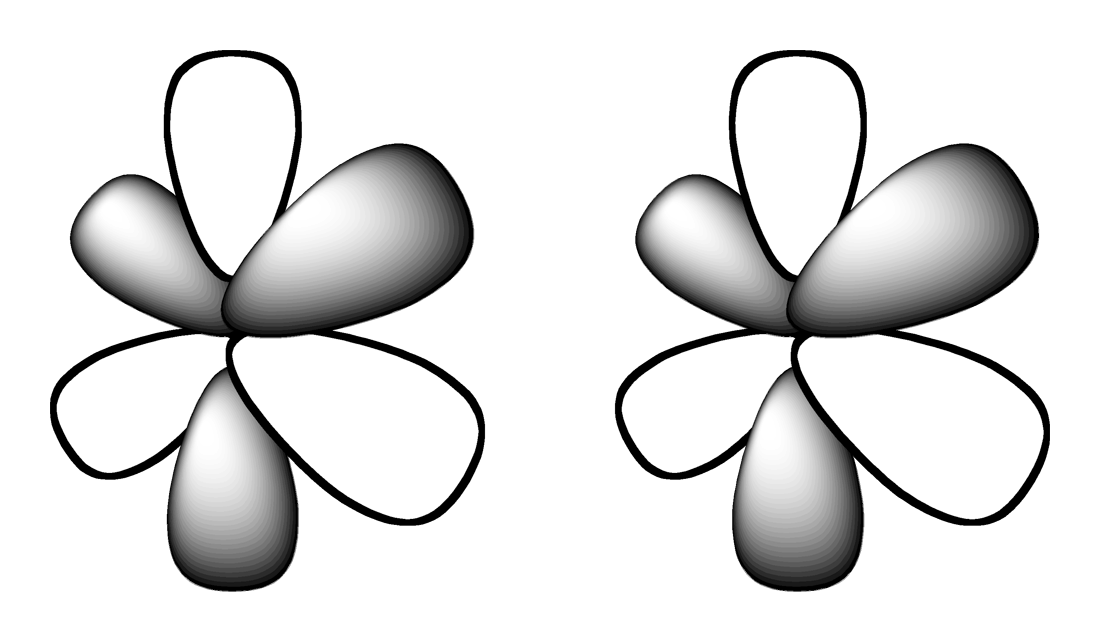
3.
OPEN ENDED QUESTION
1 min • 1 pt

Compare and contrast the Bohr Model and the Quantum Model of the atom. Describe at least two key similarities and at least one key difference.
Evaluate responses using AI:
OFF
4.
MULTIPLE SELECT QUESTION
1 min • 1 pt
There are four different orbital shapes. Select them from the following list.
"s"
"f"
"g"
"d"
"p"
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
In the quantum mechanical model of the atom, where are electrons most likely to be found?
Moving in fixed orbits around the nucleus
Embedded within the nucleus
In specific regions called electron clouds or orbitals
Uniformly distributed around the nucleus
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
In the quantum-mechanical model of the atom, an orbital is defined as a
region of the most probable proton location.
region of the most probable electron location.
circular path traveled by an electron around an orbital.
circular path traveled by a proton around an orbital.
Answer explanation
In the quantum-mechanical model of the atom, an orbital is defined as a region of the most probable electron location.
7.
MULTIPLE SELECT QUESTION
1 min • 1 pt
What is the fundamental difference between the Bohr model of an atom and the quantum mechanical model? Select all that apply.
Bohr model considers electron to be a particle; quantum mechanics considers it to be a wave.
Bohr model describes electron with 1 quantum number; quantum model uses 4 quantum.
Bohr model describes electrons in orbits; quantum model describes electrons in orbitals.
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

20 questions
Energy Sources
Quiz
•
7th - 10th Grade

20 questions
Chapter 5 Test- Biomes
Quiz
•
11th Grade

20 questions
Year 10 Science - Motion and forces quiz
Quiz
•
10th Grade

20 questions
Cell Organelles
Quiz
•
8th - 10th Grade

22 questions
Unit 2 - Matter - Practice Test (Minor Grade)
Quiz
•
12th Grade

20 questions
U4 AOS 1 - Sleep and Consciousness Part 1
Quiz
•
12th Grade

20 questions
Water Cycle
Quiz
•
9th Grade

20 questions
GRADE 6 FE 2025
Quiz
•
6th Grade - University
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Science

10 questions
Exploring Newton's Laws of Motion
Interactive video
•
6th - 10th Grade

10 questions
Unit 2: LS.Bio.1.5-LS.Bio.2.2 Power Vocab
Quiz
•
9th - 12th Grade

10 questions
Exploring Light and Waves Concepts
Interactive video
•
6th - 10th Grade

12 questions
Endothermic and Exothermic Reactions
Quiz
•
7th - 10th Grade

10 questions
Exploring Thermal Energy and Temperature Concepts
Interactive video
•
6th - 10th Grade

10 questions
Exploring Kinetic and Potential Energy Concepts
Interactive video
•
6th - 10th Grade

32 questions
Explore Mixtures and Solutions
Quiz
•
9th - 12th Grade

15 questions
Patterns of Evolution MCQ Reading
Passage
•
9th - 12th Grade
