Molecular compounds
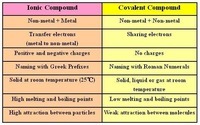
Naming Ionic and Covalent Compounds and Balancing Equations
Quiz
•
Other Sciences
•
KG - University
•
Hard

Charles Martinez
FREE Resource
12 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
share electrons
give electrons
take electrons
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Molecular compounds are made of
two non-metals
two metals
one metal and one non-metal
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Ionic compounds are made of
two non-metals
two metals
one metal and one non-metal
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
An ionic compound of calcium and chlorine would be named
calcium chlorine
calcium chloride
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
A covalent compound made of one sulfur and two oxygen atoms would be named
sulfur dioxide.
sulfur oxide.
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
An ionic compound made of magnesium and oxygen would be named
magneside oxygen.
magnesium oxide.
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
The covalent compound N2O5 would be named
dinitrogen oxide.
dinitrogen pentoxide.
nitrogen pentoxide.
Create a free account and access millions of resources
Similar Resources on Quizizz

10 questions
Naming Ionic and Covalent Compounds
Quiz
•
KG - University

10 questions
Naming Ionic and Covalent Compounds and Balancing Equations
Quiz
•
KG - University

10 questions
Naming Compounds and Bonding
Quiz
•
KG - University

10 questions
Writing and Naming Binary Covalent and Ionic Compounds
Quiz
•
KG - University

10 questions
Naming Ionic and Covalent Compounds
Quiz
•
KG - University

10 questions
Naming Ionic and Covalent Compounds
Quiz
•
KG - University

15 questions
Test 5 review
Quiz
•
9th - 11th Grade

13 questions
Atomic Structure
Quiz
•
8th Grade
Popular Resources on Quizizz

15 questions
Multiplication Facts
Quiz
•
4th Grade

25 questions
SS Combined Advisory Quiz
Quiz
•
6th - 8th Grade

40 questions
Week 4 Student In Class Practice Set
Quiz
•
9th - 12th Grade

40 questions
SOL: ILE DNA Tech, Gen, Evol 2025
Quiz
•
9th - 12th Grade

20 questions
NC Universities (R2H)
Quiz
•
9th - 12th Grade

15 questions
June Review Quiz
Quiz
•
Professional Development

20 questions
Congruent and Similar Triangles
Quiz
•
8th Grade

25 questions
Triangle Inequalities
Quiz
•
10th - 12th Grade
