
Lewis Dot Structure Practice
Authored by Evelyn Romero
Chemistry
10th Grade
NGSS covered
Used 70+ times

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

Which of the following is the correct Lewis dot structure for the molecule fluorine (F2)?
2.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt

Which of the following is the correct Lewis structure for the compound PBr3?
structure A
structure B
structure C
structure D
Tags
NGSS.HS-PS1-2
NGSS.HS-PS1-1
3.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
What is the correct Lewis structure for NH3?
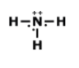
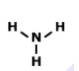
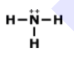
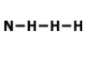
Tags
NGSS.HS-PS1-2
NGSS.HS-PS1-1
4.
MULTIPLE CHOICE QUESTION
3 mins • 1 pt
Which is the correct Lewis structure for carbon dioxide?




Tags
NGSS.HS-PS1-2
5.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt

What is the correct formula for this molecule?
Tags
NGSS.MS-PS1-1
6.
MULTIPLE SELECT QUESTION
2 mins • 1 pt

Why is this Lewis Structure incorrect? Select all that appy.
There should be a single bond between H and C.
Nitrogen does not have a fulfilled octet
Carbon has too many bonds around it.
Hydrogen needs more electrons.
Tags
NGSS.HS-PS1-2
7.
MULTIPLE SELECT QUESTION
2 mins • 1 pt

Why is this Lewis Structure incorrect? Choose all that apply.
the number of valence electrons is not correct
There should only be single bonds in this Lewis Structure.
The Structure is missing a triple bond.
Chlorine only has 6 electrons surrounding it.
Tags
NGSS.HS-PS1-2
NGSS.HS-PS1-1
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

15 questions
S1-Q4-U4 - L1 all
Quiz
•
10th Grade

11 questions
Atoms
Quiz
•
9th - 10th Grade

10 questions
Types of Electrodes
Quiz
•
10th - 12th Grade

10 questions
Alkenes
Quiz
•
9th - 10th Grade

15 questions
Sec 3 Pure Chem ECM Quiz
Quiz
•
9th - 12th Grade

10 questions
Strength of Acids and Alkalis
Quiz
•
10th Grade

10 questions
Which is it? (Biomolecules)
Quiz
•
10th Grade

15 questions
Trends of the periodic table
Quiz
•
10th Grade
Popular Resources on Wayground

7 questions
History of Valentine's Day
Interactive video
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

15 questions
Valentine's Day Trivia
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 8 Stoichiometry Review
Quiz
•
10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

19 questions
Stoichiometry, Limiting Reactants, and Percent Yield
Quiz
•
10th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

10 questions
Identifying types of reactions
Quiz
•
9th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade
