
Acids and Bases Strengths
Quiz
•
Chemistry
•
11th - 12th Grade
•
Hard
Standards-aligned

Charles Martinez
FREE Resource
20 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

Which of the following best approximates the Ka value for this weak acid?
1 x 10–3
1 x 10–4
1 x 10–5
1 x 10–6
2.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which of the following best represents a solution of H2SO4 in water?
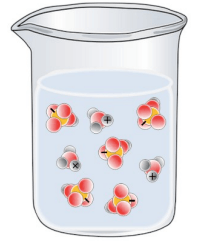
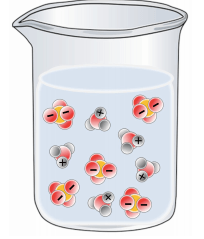
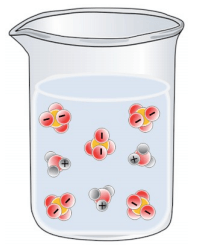
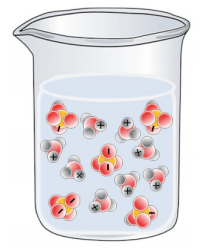
Tags
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
HSO4¯ + H2O ↔ H3O+ + SO4 2¯ In the equilibrium represented above, the species that act as bases include which of the following?
HSO4-
H2O
SO4 2¯
H2O and SO4 2¯
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Given the equation:
H2SO4 + H2O ↔ H3O+ + HSO4- 1
What is the conjugate acid?
H2SO4
H2O
H3O+
HSO4- 1
5.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which compound is the conjugate base?
HCO3- + HCl ==> H2CO3 + Cl-
Which compound is the conjugate base?
HCO3-
HCl
H2CO3
Cl-
6.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Which of the following is transferred between a conjugate acid-base pair?
an electron
a proton
a neutron
a OH- ion
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When a hydrogen ion is lost from an acid, the resulting particle is called its conjugate base. Which of the following particles would be the conjugate base for the acid HSO4–?
H2SO4
SO42–
SO4–
HSO4
Create a free account and access millions of resources
Similar Resources on Wayground

15 questions
Acids & Bases
Quiz
•
9th - 12th Grade
![Acids and Bases and Calculating Log[h+]](https://cf.quizizz.com/image/image-loader.svg)
15 questions
Acids and Bases and Calculating Log[h+]
Quiz
•
9th - 12th Grade

22 questions
AP Chemistry - Acids and Bases and pHs, oh my!
Quiz
•
11th Grade - University

24 questions
Acid Base AP Chem Review
Quiz
•
11th Grade - University

20 questions
Strength of Acids and Bases Worksheet
Quiz
•
11th - 12th Grade

20 questions
Chemistry Ch 14 - acids and bases
Quiz
•
10th - 11th Grade

17 questions
Acids and Bases
Quiz
•
7th - 12th Grade

19 questions
Acid / Base Review
Quiz
•
12th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Naming Covalent Compounds
Quiz
•
11th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

15 questions
Electron Configurations and Orbital Notation
Quiz
•
11th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

18 questions
Ions
Quiz
•
9th - 12th Grade

15 questions
Lewis Dot Structures
Quiz
•
9th - 12th Grade

71 questions
Periodicity Test Prep
Quiz
•
9th - 12th Grade
