
Science TEKS
Authored by Charles Martinez
Science
8th Grade
NGSS covered

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
18 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt

Which value is most likely the number of protons for saturntonium?
388
219
169
171
Tags
NGSS.MS-PS1-1
2.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt

Which statement is a valid conclusion based on the information?
Saturntonium is nonreactive
Saturntonium has properties unlike any other element
Saturntonium is very reactive
Saturntonium has properties most similar to period 7 metals
Tags
NGSS.MS-PS1-1
NGSS.MS-PS1-2
3.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt

What information from the table could help the student identify the element?
Protons
Neutrons
Atomic mass
Valence electrons
Tags
NGSS.MS-PS1-1
4.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Which model represents the most reactive atom?
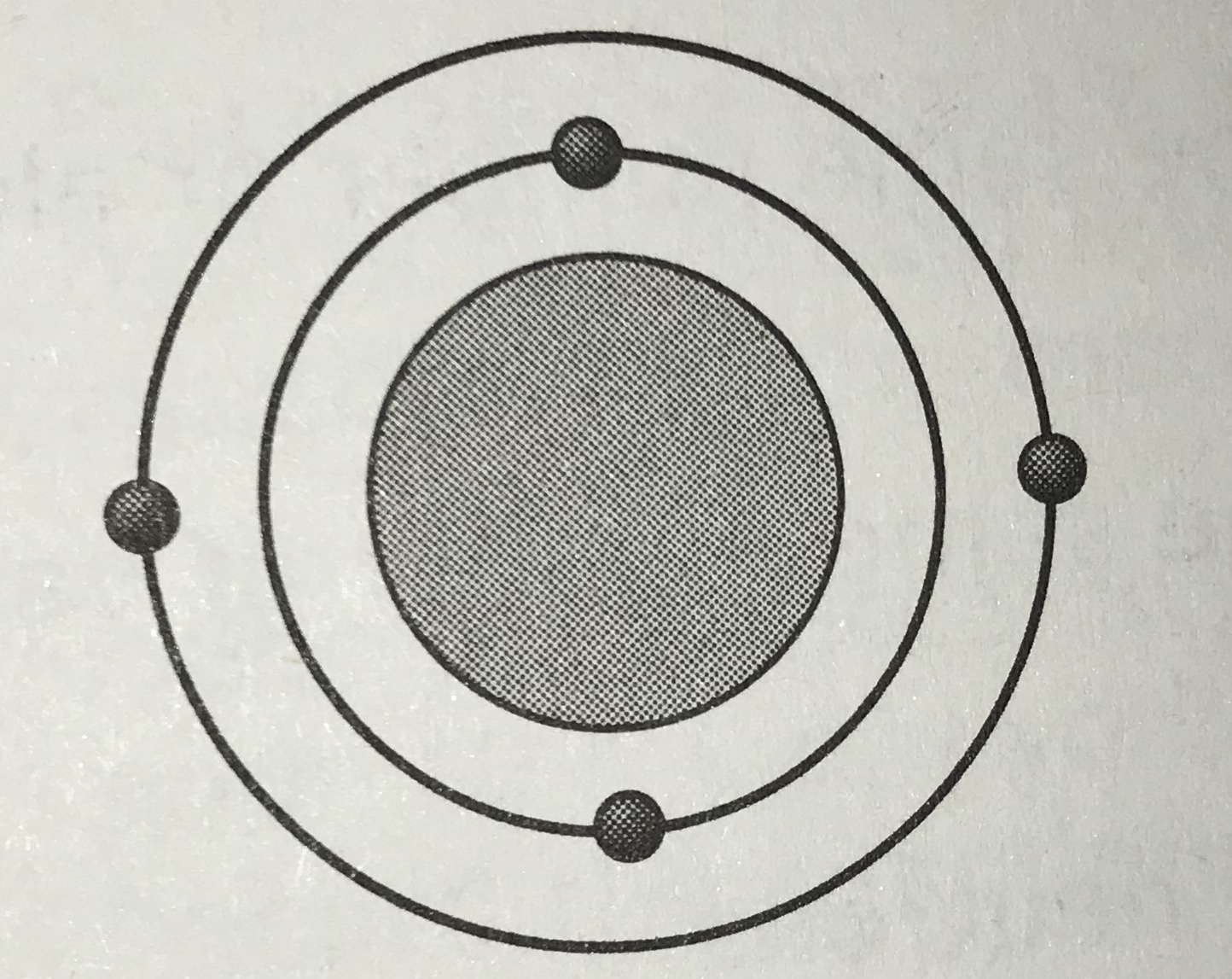
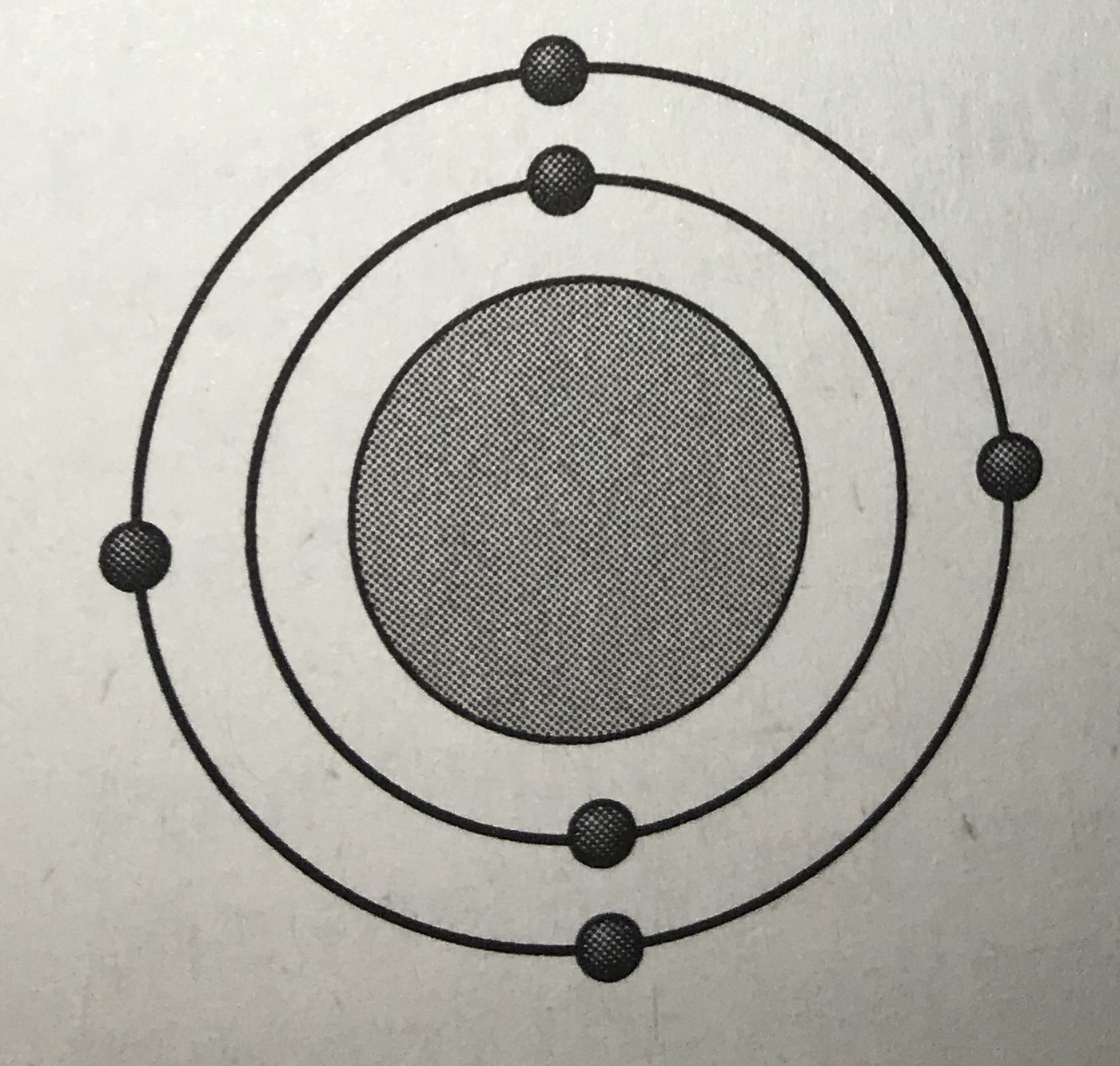
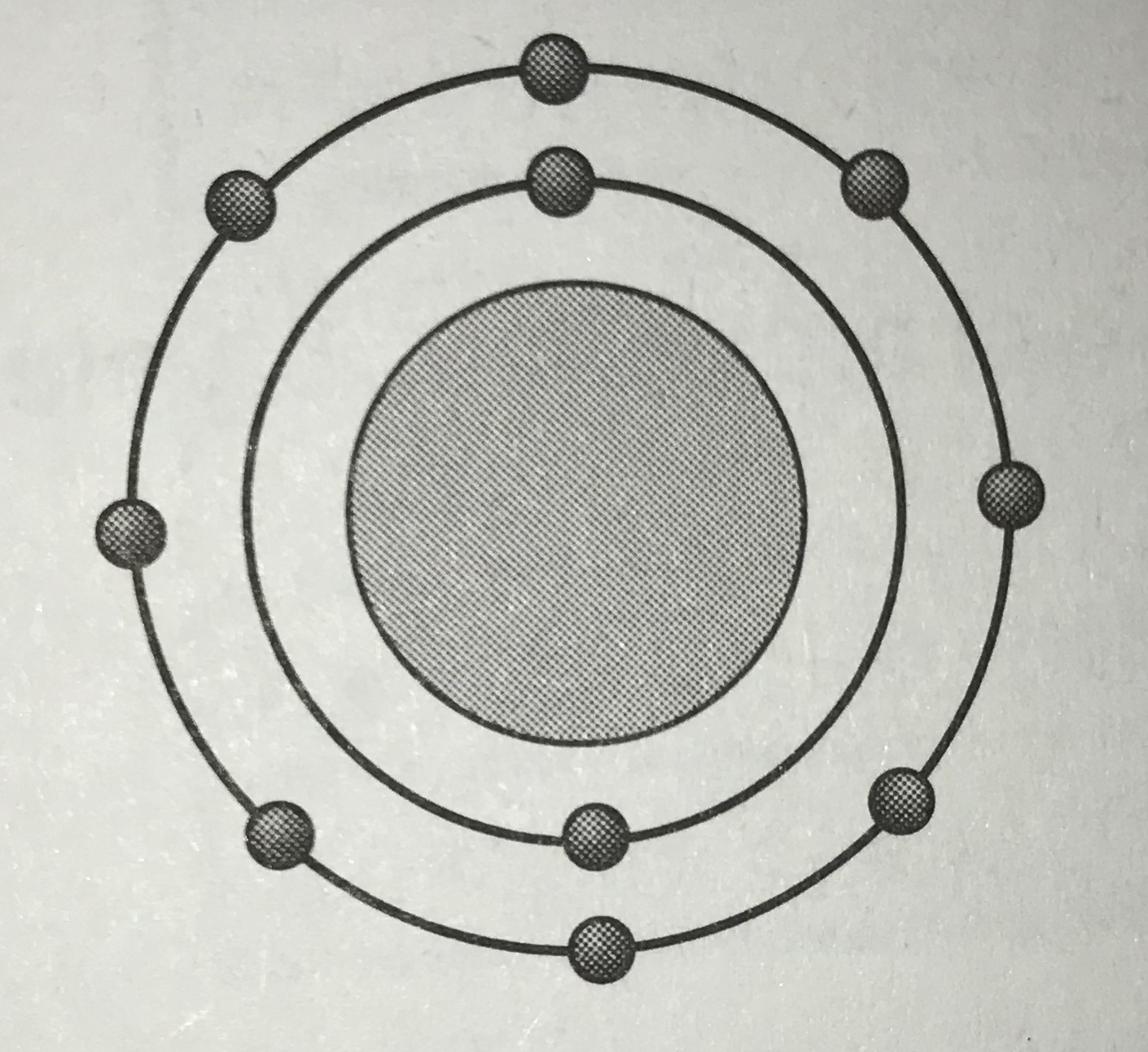
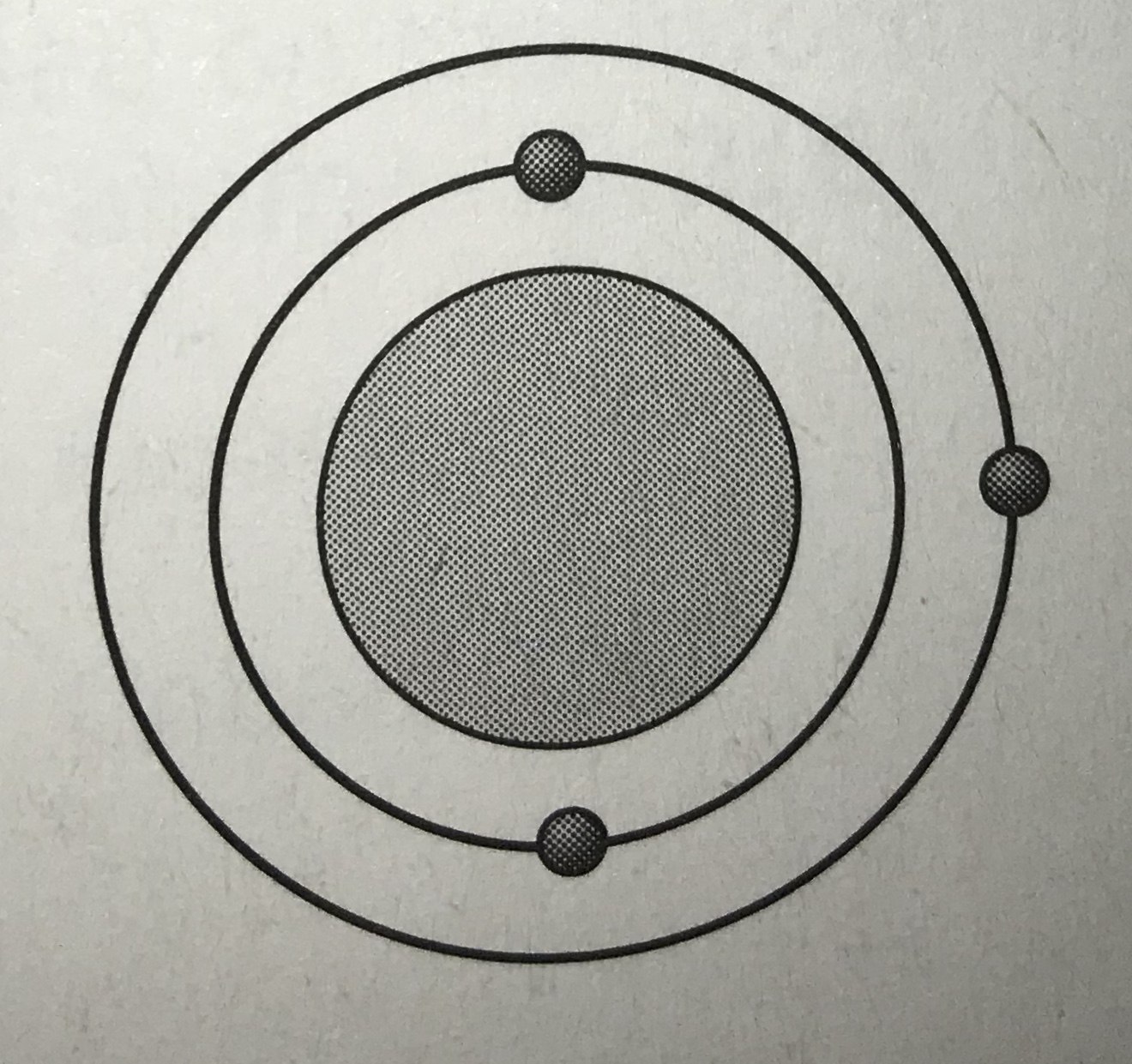
Tags
NGSS.MS-PS1-1
5.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt

What element, based on the subatomic particles in the atom, is shown?
Beryllium
Helium
Lithium
Oxygen
Tags
NGSS.MS-PS1-1
6.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
Which of the following characteristics is similar for fluorine and iodine?
Number of protons
Number of neutrons
Number of orbitals
Number of valence electrons
7.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
What element contains 7 protons and 5 valence electrons?
Boron
Vanadium
Nitrogen
Lithium
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

20 questions
Simple Machines: First Class Levers
Quiz
•
6th - 8th Grade

14 questions
British Science Week 2021
Quiz
•
8th Grade

17 questions
Elements, Compounds, and Valence Electrons
Quiz
•
8th Grade

20 questions
Changes in States of Matter
Quiz
•
8th Grade

14 questions
Day and Night 1
Quiz
•
6th - 9th Grade

14 questions
UNIT 1 REVIEW
Quiz
•
8th Grade

20 questions
Astronomy by Sakarya Bilsem
Quiz
•
3rd - 8th Grade

20 questions
Matter: Lessons 5, 6, 7 (in book)
Quiz
•
5th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

10 questions
Probability Practice
Quiz
•
4th Grade

15 questions
Probability on Number LIne
Quiz
•
4th Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

6 questions
Appropriate Chromebook Usage
Lesson
•
7th Grade

10 questions
Greek Bases tele and phon
Quiz
•
6th - 8th Grade
Discover more resources for Science

10 questions
Exploring the Rock Cycle
Interactive video
•
6th - 8th Grade

8 questions
Newton's Second Law
Lesson
•
6th - 8th Grade

8 questions
Amoeba Sister Asexual vs Sexual Reproduction
Interactive video
•
8th Grade

10 questions
Exploring the Rock Cycle: Types and Formation
Interactive video
•
6th - 8th Grade

20 questions
Plate Tectonics
Quiz
•
8th Grade

25 questions
Evolution and Natural Selection Review
Quiz
•
6th - 8th Grade

10 questions
Exploring Weathering, Erosion, and Deposition Processes
Interactive video
•
6th - 10th Grade

20 questions
Rock Cycle
Quiz
•
8th Grade