
Rate of Reaction
Quiz
•
Science
•
10th Grade
•
Hard
Standards-aligned

Charles Martinez
FREE Resource
25 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Why does a higher temperature increase the rate of a reaction?
it increases both the frequency and energy of particle collisions
it only increases the frequency of particle collisions
it only increases the energy of particle collisions
it reduces the activation energy of the reaction
Tags
NGSS.HS-PS1-5
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Grinding a effervescent tablet into powder increases the rate of reaction due to increased
concentration
surface area
temperature
reactants
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Adding a catalyst ___________ the activation energy.
Raises
Lowers
Doesn't affect
Inreases
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
The minimum amount of energy needed for colliding particles to react is called
Chemical Energy
Kinetic Energy
Activation Energy
Potential Energy
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which graph shows the effect of increasing temperature on the rate of reaction of calcium carbonate with dilute hydrochloric acid?
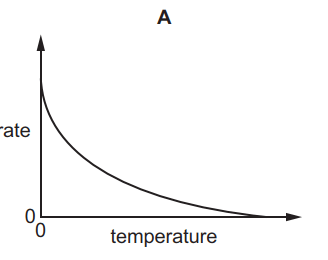
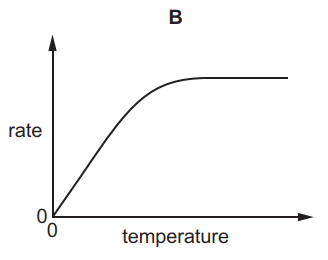
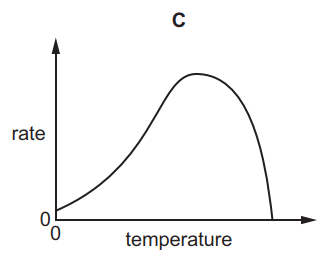
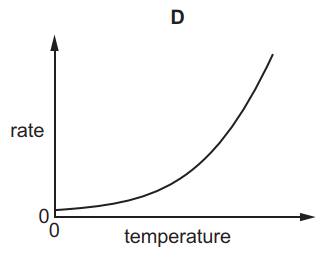
Tags
NGSS.HS-PS1-5
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Tags
NGSS.HS-PS1-5
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Tags
NGSS.HS-PS1-5
Create a free account and access millions of resources
Similar Resources on Wayground

20 questions
Reversible Irreversible Reactions Catalyst Collision Theory
Quiz
•
9th - 11th Grade

20 questions
Biology vocab
Quiz
•
9th - 12th Grade

22 questions
Types of Chemical Reactions
Quiz
•
9th - 10th Grade

20 questions
Equilibrium and Equilibrium Expressions
Quiz
•
10th - 12th Grade

21 questions
Chemical Reactions Quiz
Quiz
•
10th Grade

25 questions
Chemistry of Life
Quiz
•
9th - 12th Grade

21 questions
Chemical reactions
Quiz
•
7th - 10th Grade

22 questions
Equilibrium Chemistry
Quiz
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Science

10 questions
Exploring the Scientific Method
Interactive video
•
6th - 10th Grade

10 questions
Exploring Chemical and Physical Changes
Interactive video
•
6th - 10th Grade

10 questions
Exploring the Basics of Density
Interactive video
•
6th - 10th Grade

10 questions
Kinetic and Potential Energy Explained
Interactive video
•
6th - 10th Grade

10 questions
The Evolution of Atomic Theory
Interactive video
•
6th - 10th Grade

10 questions
Exploring Biomes and Ecosystems for Kids
Interactive video
•
6th - 10th Grade

10 questions
Exploring Metals, Nonmetals, and Metalloids
Interactive video
•
6th - 10th Grade

10 questions
Exploring Latitude and Longitude Concepts
Interactive video
•
6th - 10th Grade


