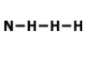Covalent Bonding, VSEPR, and Polarity Lesson
Quiz
•
Chemistry
•
9th - 12th Grade
•
Hard
Standards-aligned

Charles Martinez
FREE Resource
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

CO2 has polar bonds but is a NON POLAR molecule. Why?
it has an asymmetrical shape
bond polarity or dipole moment between C and O atoms cancel
there is net dipole moment between C and O atoms in molecule
bond polarity does not exist between C+ and O- atoms
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Does the following reference Polar, Nonpolar, or both:
"electronegativity values are the same"?
Polar
Nonpolar
Both
Tags
NGSS.HS-PS1-1
3.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt

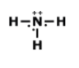
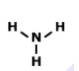
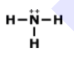
Is this molecule polar or nonpolar?
polar
nonpolar
Tags
NGSS.HS-PS1-2
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
6.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Type of bond between Br & Br
Ionic
Polar Covalent
Nonpolar Covalent
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Create a free account and access millions of resources
Similar Resources on Wayground

16 questions
Unit 6 VocabTest
Quiz
•
10th Grade

20 questions
Molecular Polarity
Quiz
•
10th Grade

16 questions
Covalent Bonding, Molecular Geometry and Polarity Lesson
Quiz
•
9th - 12th Grade

16 questions
Molecular Shapes and Polarity
Quiz
•
9th - 12th Grade

16 questions
Molecular Geometry and Polarity
Quiz
•
9th - 12th Grade

12 questions
Molecular Geometry Lesson
Quiz
•
9th - 12th Grade

15 questions
Lewis Dot Structure, Geometry, Polarity
Quiz
•
9th - 12th Grade

15 questions
Basics of IMF
Quiz
•
8th - 12th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

20 questions
Naming Covalent Compounds
Quiz
•
11th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade