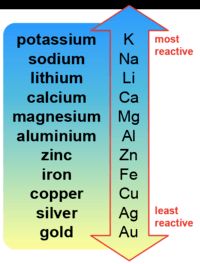
Igcse Science Reactivity Series
Quiz
•
Chemistry
•
8th - 10th Grade
•
Easy
+1
Standards-aligned

Charles Martinez
Used 17+ times
FREE Resource
14 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Which of the following is MOST reactive?
Magnesium
Lead
Tin
Copper
Zinc
2.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Which metal is more reactive than calcium?
magnesium
potassium
silver
aluminum
3.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Tags
NGSS.MS-PS1-2
4.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Choose the least reactive metal
calcium
magnesium
iron
copper
5.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Choose the most reactive metal
calcium
magnesium
iron
copper
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
6.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
Tags
NGSS.MS-PS1-2
7.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt
What does displacement mean?
A chemical reaction where a less reactive metal takes the place of a more reactive metal in a compound.
A chemical reaction where a more reactive metal takes the place of a less reactive metal in a compound.
A more reactive metal is removed from a compound.
Create a free account and access millions of resources
Similar Resources on Wayground

14 questions
Ch 12: Metals
Quiz
•
10th Grade

10 questions
Ionic Compounds Quiz (Q2W2)
Quiz
•
10th Grade

10 questions
Reactivity Series
Quiz
•
8th - 10th Grade

10 questions
Naming Ionic Compounds
Quiz
•
10th Grade

15 questions
ATS KS4 Chemistry: Electrolysis
Quiz
•
10th - 12th Grade

11 questions
Electrolysis
Quiz
•
10th - 11th Grade

10 questions
Reactivity of Metals
Quiz
•
8th - 10th Grade

10 questions
Oxidation and Reduction
Quiz
•
8th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Physical and Chemical Properties
Quiz
•
8th Grade

20 questions
States of Matter
Quiz
•
8th Grade

20 questions
Counting Atoms Practice
Quiz
•
8th Grade

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

20 questions
Chemical Reactions
Quiz
•
8th Grade

15 questions
Periodic Table of Elements
Quiz
•
8th Grade



