
Lewis Structures and Molecular Nomenclature
Quiz
•
Chemistry
•
10th Grade
•
Hard
+1
Standards-aligned
Travis McClellan
FREE Resource
29 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
What is the correct Lewis Dot Structure for ammonia NH3
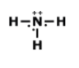
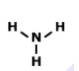
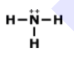
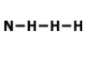
Tags
NGSS.HS-PS1-1
2.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
Tags
NGSS.HS-PS1-1
4.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt

Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
5.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt

Tags
NGSS.HS-PS1-3
6.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
7.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
Create a free account and access millions of resources
Similar Resources on Wayground

33 questions
Binary Ionic Compounds Names and Formulas Practice
Quiz
•
9th - 12th Grade

24 questions
Igcse Ionic and Covalent Lewis Dot Structures
Quiz
•
10th - 11th Grade

24 questions
Ionic and Covalent
Quiz
•
10th - 11th Grade

24 questions
Ionic or Covalent
Quiz
•
10th - 11th Grade

24 questions
Simple Binary Ionic and Covalent Bonds
Quiz
•
10th - 11th Grade

24 questions
Acids, Ionics, and Covalents
Quiz
•
10th - 11th Grade

26 questions
LG05 - Particle View Diagrams Review
Quiz
•
9th - 12th Grade

24 questions
Bonding & Lewis Structures
Quiz
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade


