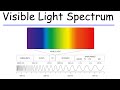
Photon Energy and Light Properties
Interactive Video
•
Physics, Science
•
9th - 12th Grade
•
Easy
Mia Campbell
Used 9+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which color of light has the longest wavelength?
Green
Yellow
Blue
Red
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the wavelength of red light approximately?
300 nm
500 nm
690 nm
400 nm
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which color of light has the highest frequency?
Red
Yellow
Green
Blue
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formula to calculate frequency from wavelength?
f = c / λ
f = c * λ
f = λ / c
f = λ * c
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the frequency of a photon with a wavelength of 460 nm?
5.00 x 10^14 Hz
6.52 x 10^14 Hz
3.00 x 10^8 Hz
1.00 x 10^12 Hz
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is Planck's constant value used in energy calculations?
6.626 x 10^-34 J·s
3.00 x 10^8 m/s
1.60 x 10^-19 J
9.81 m/s^2
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How much energy does a photon of blue light with a wavelength of 460 nm have in electron volts?
1.5 eV
2.7 eV
2.0 eV
3.0 eV
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Popular Resources on Wayground

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

20 questions
Halloween Trivia
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

4 questions
Activity set 10/24
Lesson
•
6th - 8th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
How to Email your Teacher
Quiz
•
Professional Development

15 questions
Order of Operations
Quiz
•
5th Grade

30 questions
October: Math Fluency: Multiply and Divide
Quiz
•
7th Grade
Discover more resources for Physics

11 questions
Speed - Velocity Comparison
Interactive video
•
9th - 12th Grade

14 questions
Bill Nye Waves
Interactive video
•
9th - 12th Grade

25 questions
Newton's Laws of Motion
Quiz
•
9th Grade

13 questions
Energy Transformations
Quiz
•
10th Grade

20 questions
Force Concept Review
Quiz
•
9th - 12th Grade

10 questions
Types of Chemical Reactions
Quiz
•
10th Grade

10 questions
Newton's Third Law
Quiz
•
7th - 11th Grade

20 questions
Calculating Net Force
Quiz
•
6th - 9th Grade