Exploring Lewis Dot Structures
Interactive Video
•
Chemistry
•
6th - 10th Grade
•
Practice Problem
•
Hard
Olivia Brooks
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does potassium have?
3
2
4
1
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the electron configuration of potassium?
2-8-8-1
2-8-8-2
2-8-7-2
2-7-8-1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons do both oxygen and sulfur have?
7
4
5
6
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the electron configuration of sulfur?
2-6
2-7-6
2-8-6
2-8-7
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
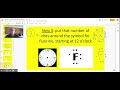
Where should you start placing dots when drawing a Lewis dot structure?
3 o'clock
6 o'clock
12 o'clock
9 o'clock
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does fluorine have?
8
6
5
7
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the correct Lewis dot structure for nitrogen?
N with 4 dots
N with 5 dots
N with 6 dots
N with 3 dots
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Counting Atoms
Quiz
•
8th Grade

20 questions
Energy Transformations
Quiz
•
9th - 12th Grade

24 questions
Chemical changes
Quiz
•
8th Grade

20 questions
Periodic Table & Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

24 questions
Identifying Types of Chemical Reactions
Quiz
•
10th - 12th Grade

13 questions
Bill Nye - Energy
Interactive video
•
6th Grade