
Rates of Reaction
Quiz
•
Chemistry
•
University
•
Practice Problem
•
Easy

Tristen Greenwood
Used 2+ times
FREE Resource
Enhance your content in a minute
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Why does a higher temperature increase the rate of a reaction?
it increases both the frequency and energy of particle collisions
it only increases the frequency of particle collisions
it only increases the energy of particle collisions
it reduces the activation energy of the reaction
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Grinding a effervescent tablet into powder increases the rate of reaction due to increased
concentration
surface area
temperature
reactants
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Adding a catalyst ___________ the activation energy.
Raises
Lowers
Doesn't affect
Inreases
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
The minimum amount of energy needed for colliding particles to react is called
Chemical Energy
Kinetic Energy
Activation Energy
Potential Energy
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which graph shows the effect of increasing temperature on the rate of reaction of calcium carbonate with dilute hydrochloric acid?
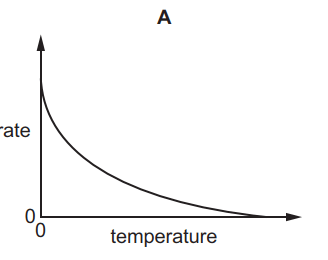
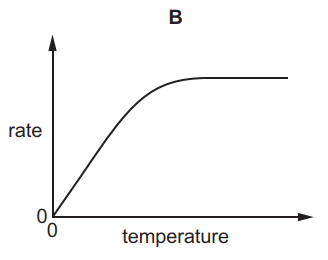
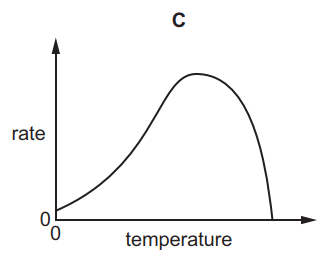
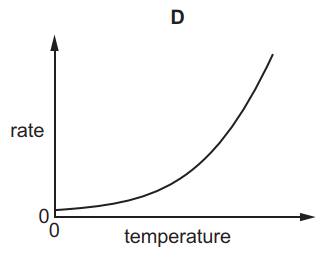
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Why does a catalyst increase the rate of reaction?
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Why don't all collisions between particles cause a reaction?
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

10 questions
OCR C2 Chemistry
Quiz
•
KG - University

15 questions
Hair Care Products
Quiz
•
University - Professi...

10 questions
FREE ENERGY
Quiz
•
10th Grade - University

11 questions
PAPA 321.3: Volumetric Analysis
Quiz
•
12th Grade - University

10 questions
Pollution
Quiz
•
12th Grade - University

10 questions
Anime
Quiz
•
KG - University

10 questions
Synthesis Reactions
Quiz
•
9th Grade - University

10 questions
Environmental chemistry
Quiz
•
12th Grade - University
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade

