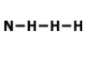Bonding Unit Review
Quiz
•
Science
•
10th Grade
•
Easy
+1
Standards-aligned
Ryan Humphrey
Used 2+ times
FREE Resource
Enhance your content in a minute
49 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 5 pts
What two types of atoms make a covalent bond?
metal atom and metal atom
metal atom and non metal non atom
non metal atom and non metal atom
2.
MULTIPLE CHOICE QUESTION
30 sec • 5 pts
What part of an atom is involved in chemical bonding?
proton
neutron
electron
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
30 sec • 5 pts
Predict the bond that is formed between Phosophorus and Chlorine?
Ionic
Covalent
Metallic
H-bond
Tags
NGSS.HS-PS1-1
4.
MULTIPLE CHOICE QUESTION
30 sec • 5 pts

How many valence electrons are shown in the diagram?
16
7
6
2
Tags
NGSS.HS-PS1-1
5.
MULTIPLE CHOICE QUESTION
30 sec • 5 pts

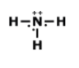
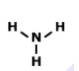
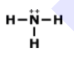
Examine the image: How many bonds are formed looking at the model of the compound? And also indetify what type of bond is formed.
2 bonds; covalent
4 bonds; metallic
2 bonds; ionic
4 bonds; covalent
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
6.
MULTIPLE CHOICE QUESTION
30 sec • 5 pts
This refers to the tendency of atoms to prefer to have eight electrons in the valence shell
Octet rule
Valence electron
Electronegativity
Lewis symbol
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
7.
MULTIPLE CHOICE QUESTION
30 sec • 5 pts

Name this element.
Neon
Magnesium
Sodium
Manganese
Tags
NGSS.HS-PS1-1
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

54 questions
Renewable and Nonrenewable resources
Quiz
•
9th Grade - University

54 questions
Environmental Science Final Review
Quiz
•
9th - 12th Grade

51 questions
12. THE UNIVERSE
Quiz
•
9th - 12th Grade

44 questions
Unit 2 - Atoms & the Periodic Table
Quiz
•
10th Grade

50 questions
Biogeochemical Cycles
Quiz
•
9th - 12th Grade

50 questions
Energy, Insolation & Seasons Review
Quiz
•
10th Grade

50 questions
Calday The Heart & Blood Vessels
Quiz
•
9th - 11th Grade

50 questions
Skeletal Muscle Fibers and Muscle contraction notes review
Quiz
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

6 questions
FOREST Self-Discipline
Lesson
•
1st - 5th Grade

7 questions
Veteran's Day
Interactive video
•
3rd Grade

20 questions
Weekly Prefix check #2
Quiz
•
4th - 7th Grade
Discover more resources for Science

10 questions
Exploring Newton's Laws of Motion
Interactive video
•
6th - 10th Grade

50 questions
Review for Test 2: HS LS 1-1
Quiz
•
9th - 12th Grade

12 questions
Endothermic and Exothermic Reactions
Quiz
•
7th - 10th Grade

16 questions
CFA #1 - Chapters 16.1, 16.2
Quiz
•
9th - 12th Grade

10 questions
Newton's Laws in NFL Action
Interactive video
•
6th - 10th Grade

10 questions
Exploring Earth's Spheres and Their Interactions
Interactive video
•
6th - 10th Grade

10 questions
Exploring the Electromagnetic Spectrum
Interactive video
•
6th - 10th Grade

10 questions
Exploring the Law of Conservation of Energy
Interactive video
•
6th - 10th Grade