
Rate of Reactions
Quiz
•
Chemistry
•
9th - 11th Grade
•
Hard
+2
Standards-aligned
Adem Koc
Used 42+ times
FREE Resource
25 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
20 sec • 1 pt
What is the meaning of the rate of reaction?
Decrease in amount of product
Decrease in amount of product against time
Increase in amount of products against time
Increase in amount of reactants against time
Tags
NGSS.HS-PS1-5
2.
MULTIPLE CHOICE QUESTION
20 sec • 1 pt
The following equation represents a chemical equation.
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
Which graph shows the correct change in mass of reactant used in excess against time?


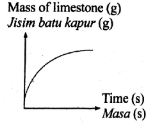
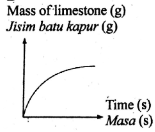
Tags
NGSS.HS-PS1-7
3.
MULTIPLE CHOICE QUESTION
20 sec • 1 pt
The rate of reaction for the decomposition of hydrogen peroxide decreases with time because ...
concentration of water decreases
temperature of hydrogen peroxide decreases
volume of hydrogen peroxide decreases
concentration of hydrogen peroxide decreases
Tags
NGSS.HS-PS1-5
4.
MULTIPLE CHOICE QUESTION
20 sec • 1 pt
In which of the chemical reactions can the rate be determined by measuring the change in the gas volume?
oxygen gas reacting with hydrogen gas
Sodium hydroxide solution with dilute hydrochloric acid
Silver nitrate solution with sodium chloride solution
Calcium carbonate with dilute hydrochloride acid
Tags
NGSS.HS-PS1-5
5.
MULTIPLE CHOICE QUESTION
20 sec • 1 pt
The reaction between zinc, Zn and hydrochloric acid, HCl is represented by the following equation.
Zn + 2HCl --> ZnCl2 + H2
A student wants to determine the rate of reaction in a school laboratory. Which of the following methods is the most suitable?
Determine the change in temperature of the solution by time
Determine the change in the concentration of zinc chloride by time
Determine the volume of hydrogen gas given off by time
Determine the change in the concentration of hydrochloric acid by time
Tags
NGSS.HS-PS1-5
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
The following equation represents the reaction between calcium carbonate, CaCO3 and hydrochloric acid, HCl.
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
Which changes can be used to determine the rate of reaction?
I : mass of calcium carbonate per unit time
II : Volume of carbon dioxide released per unit time
III : Colour of solution per unit time
IV : Mass of precipitate produced per unit time
I and II
I and III
II and IV
III and IV
Tags
NGSS.HS-PS1-5
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

Diagram shows a graph of volume of oxygen gas collected against time in the decomposition reaction of hydrogen peroxide when using manganese dioxide as catalyst.
Which point shows the highest rate of reaction?
P
Q
R
S
Tags
NGSS.HS-PS1-5
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

20 questions
Year 9 Data Test Revision
Quiz
•
9th Grade

20 questions
Kuis Teori Asam-Basa
Quiz
•
11th Grade

20 questions
Atmosphere & Pollution Revision Test
Quiz
•
10th - 12th Grade

20 questions
quiz 2 sec 2
Quiz
•
11th Grade

20 questions
UH AKD
Quiz
•
10th Grade

20 questions
FUNDIS QUIZFIN1
Quiz
•
4th Grade - University

20 questions
Gas Laws Exam Review
Quiz
•
10th Grade

20 questions
Periodicity I
Quiz
•
10th - 12th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

14 questions
PERIODIC TRENDS
Quiz
•
11th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade
